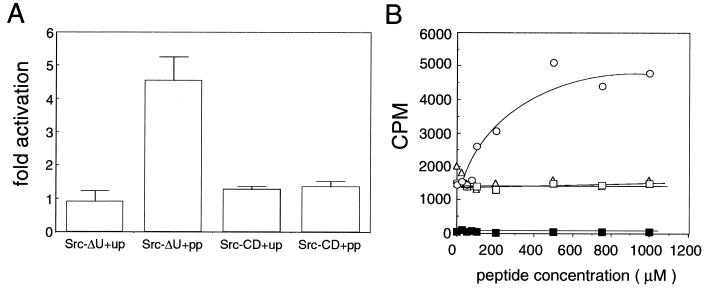
Figure 3.
Activation of Src-ΔU by phosphopeptides. (A) Influence of different peptides on the activity of Src-ΔU and Src-CD. Kinase assays using purified Src-ΔU and Src-CD were carried out in 60 μl of kinase buffer containing 100 μM substrate peptide, 0.02 μM enzyme, and 1 mM activation peptide. After 30 min of preincubation the reaction was started as described in Materials and Methods. After 2, 4, 6, and 8 min, 10-μl samples were pipetted on phosphocellulose units and washed twice with 75 mM phosphoric acid, and treated as described in Table 1. Incorporation was linear over the time range of the experiment. Fold activation for the four different time points was calculated as follows. The counts obtained in the presence of unphosphorylated (up) or phosphorylated (pp) middle T peptide were divided by the counts obtained in the absence of activating peptide. Error bars represent the standard deviation of the four time points. Counts obtained from the control experiments (without enzyme) were subtracted and corresponded to about 0.5% of the Src-CD values and 10% of the Src-ΔU values. (B) Phosphopeptide titration. Different peptides were tested for their ability to activate Src at different concentrations. Assay conditions were the same as in Fig. 3A except that reaction volumes were 20 μl and the reaction time was 3 min. Counts per minute incorporated into the substrate were plotted against the peptide concentration. Phosphorylated (○), unphosphorylated (▵), and polyoma middle T peptide or phosphorylated tail peptide (□) were included in the preincubation at different concentrations as indicated. Control reactions were carried out without enzyme (▪).