FIG. 2. Effect of astrocyte-derived TGF-β on E2- and TMX-mediated neuroprotection from camptothecin (CPT) in mixed cortical cultures.
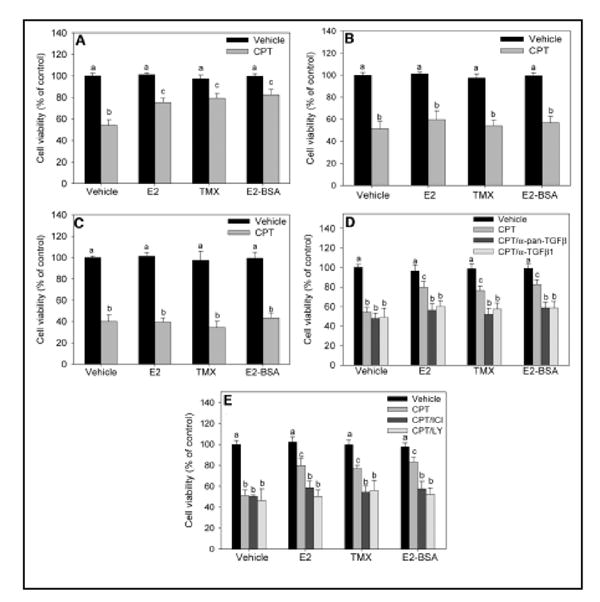
A, Effects of E2, TMX, and E2-BSA on cell death induced by CPT in mixed glial-neuronal cultures. Mixed cultures were pretreated for 24 h with 10 nM E2, 1 μM TMX, or 100 nM E2-BSA before 10 μM CPT treatment for 24 h before determination of cell viability. B, Treatment of glial-neuronal mixed cultures with E2, TMX, or E2-BSA at the time of CPT treatment does not affect cell death 24 h later. C, Pretreatment of purified cortical neurons with E2, TMX, or E2-BSA does not reverse the cell death induced by 24 h 10 μM CPT. D, Mixed cultures were pretreated with E2, TMX, or E2-BSA in the presence or absence of a pan-specific TGF-β isoform-neutralizing antibody (α-pan-TGFβ) or a TGF-β1-specific neutralizing antibody (α-TGF-β1), followed by a 24-h CPT exposure. Cell viability was determined 24 h after treatment with CPT. E, Effects of the ER antagonist, ICI182,780, and the PI3-K inhibitor, LY294002, on E2- and TMX-mediated rescue. Mixed cultures were pretreated with E2, TMX, or E2-BSA in the presence or absence of 1 μM ICI182,780 or 20 μM LY294002 for 24 h. Cultures were then exposed to CPT for another 24 h, followed by determination of cell viability. For all studies, cellular viability was determined using the MTT assay. Vehicle-treated cultures were considered to be 100% viable, and all treatment groups were compared with these control cultures. Viability was also confirmed using lactate dehydrogenase release assays (data not shown). In all panels, data are expressed as the mean ± SEM, and groups with different superscripts are significantly different from each other (P < 0.05, by one-way ANOVA, Student-Newman-Keuls post hoc test). For all studies, there were six wells per treatment group and experiments were performed in three independent sets of cultures for verification of results. Reproduced with permission from: Dhandapani, K. M. et al. Endocrinology 2005;146:2749-2759.
