Summary
Clinical relevance
Measurement of markers of cartilage pathology in synovial fluid may provide clinical rheumatologists and osteoarthritis (OA) researchers important information for early diagnosis of OA as well as a method for monitoring disease progression and response to treatment. This study demonstrates the value of this approach in an established model of OA (cranial cruciate ligament rupture) at a point distant enough from the original surgical manipulation so as to have little to no effect on the marker concentrations.
Objective
The objective of this study was to determine whether measurement of markers of cartilage collagen cleavage and proteoglycan turnover in synovial fluid from a canine model could be used to detect cartilage changes following the onset of joint instability during the development of OA.
Design
A model of joint instability that develops OA was created in 18 mature dogs using monopolar radiofrequency energy (MRFE). MRFE was arthroscopically applied to one cranial cruciate ligament (CCL) while the contralateral CCL was sham treated. The treated CCLs ruptured approximately 8 weeks (55 ± 1.6 days) after MRFE treatment. Synovial fluid was collected at time zero prior to MRFE treatment, 4 weeks after MRFE treatment, and at 4, 8, and 16 weeks after CCL rupture. Synovial fluid concentrations of the neoepitope COL2-3/4C long (type II collagen cleavage by collagenase) and epitopes 3B3(–) (proteoglycan aggrecan sulfation) and 846 (associated with aggrecan synthesis) were analyzed.
Results
Compared to sham treated joints, the synovial fluid concentrations of COL2-3/4C long and 3B3(–) were significantly increased 2.2 fold and 2.9 fold, respectively, in joints with MRFE treated CCLs following CCL rupture. Concentrations of the 846 epitope in synovial fluid showed a trend toward an increase, which was not significant, after CCL rupture.
Conclusions
Concentrations of the collagenase-cleaved type II collagen neoepitope and 3B3(–) epitope in synovial fluid were significantly increased by 4 weeks and remained elevated for at least 16 weeks after CCL rupture. This suggests that in dogs the COL2-3/4C long neoepitope and 3B3(–) epitope are sensitive markers for changes in joint cartilage turnover in joints that are developing OA.
Keywords: COL2-3/4 long, 3B3(—); osteoarthritis; monopolar radiofrequency energy; knee; canine; cranial cruciate ligament
Introduction
Osteoarthritis (OA) is characterized by the progressive degradation and erosion of articular cartilage in synovial joints1. Many studies have been conducted over the past two decades to investigate the pathogenesis and mechanisms of OA2–10. One proposed mechanism for the development of OA is that disturbances in the balance between synthesis and degradation of the extracellular cartilage matrix are the initiating events11. Cartilage matrix consists of two major macromolecules, type II collagen and the proteoglycan aggrecan. Collagen fibrils provide tensile strength to maintain tissue integrity, whereas aggrecan, interwoven with the collagen fibrils, contributes to cartilage matrix compressive stiffness12–14.
Damage to type II collagen and loss of aggrecan are fundamental features of damage to articular cartilage in OA. This is attributed to proteolytic enzymes secreted by chondrocytes and synoviocytes. Three collagenases of the matrix metalloproteinase (MMP) family—collagenase-1 (MMP-1), collagenase-2 (MMP-8), and collagenase-3 (MMP-13)—are known to be responsible for the primary cleavage of the triple helix of type II collagen15,16. Levels of collagenase-mediated cleavage16 and denaturation17 of type II collagen are increased in human osteoarthritic cartilage. The collagenases cleave triple helical collagen at a single site, resulting in a three-quarter and a one-quarter fragment. A cartilage-specific type II collagen monoclonal antibody has been developed to detect levels of the carboxy terminal neoepitope of the three-quarter-piece generated by collagenase at this primary cleavage site (M. lonescu and A. R. Poole, in preparation).
The proteoglycan (PG) aggrecan, another important component of the cartilage matrix, consists of an extended core protein to which chondroitin-sulfate and keratan sulfate glycosaminoglycans are attached9,14. Many studies have been conducted of PG in OA2–5,14, and these have shown that there are significant PG changes in the disease process. A number of antibodies have been developed over the last decade to detect epitopes reflective of PG metabolism. Of particular interest are the PG chondroitin-sulfate epitopes 3B3(–) and 846. Monoclonal antibody 3B3(–) recognizes PG bearing an epitope in native chondroitin-sulfate glycosaminoglycan chains that possess a saturated hexuronic acid residue adjacent to 6-sulfated N-acetyl galactosamine at the non-reducing terminus of the chondroitin-sulfate glycosaminoglycan3. It has been reported that the 3B3(–) epitope is not present at significant levels in normal cartilage, but increases in cartilage and synovial fluid in human OA and in experimental animal models of QA8,9. However, controversy exists about this epitope. Plass and co-workers found that most of the 3B3(–) epitope from osteoarthritic cartilage was within normal levels, with only 21% of the patients displaying elevated 3B3(–) epitope reactivity18.
Monoclonal antibody 846 recognizes a chondroitin-sulfate epitope on aggrecan that is maximally expressed in fetal cartilage19. The 846 epitope is typically almost absent from adult cartilage, but is usually increased in OA, with the most marked increases in phase II of the disease in which fibrillation is more extensive and cartilage becomes very degenerative4,5. This epitope is present on the largest molecules, and is a marker of elevated aggrecan synthesis.
Detection of elevations in the concentrations of the above neoepitope and epitopes in synovial fluid is perhaps the best way to identify subtle changes in cartilage turnover in early stages of OA. Hazell8 and his group measured the levels of synovial fluid 3B3(–) and 7D4 chondroitin-sulfate epitopes, and 5D4 keratan sulfate epitope from injured knees of patients with cruciate ligament and meniscal damage. High levels of 3B3(–) and 7D4 were found in most of the injured patients, while 5D4 epitope was decreased. Ratcliffe et al.9 also reported that 3B3(–) epitope in OA synovial fluid was elevated 33–35 fold. Epitope 846 synovial fluid levels were high in OA patients with the longest disease duration and greatest loss of cartilage5, and increased in joint fluid following injury to that joint20.
The objectives of this study were to determine synovial fluid concentrations of COL2-3/4 long neoepitope and 3B3(–) and 846 epitopes following the creation of joint instability in dogs and to monitor any subsequent changes in their concentrations. We created an instability model by applying monopolar radiofrequency energy (MRFE) arthroscopically to the cranial cruciate ligament (CCL) of the dog. Following treatment, the CCL progressively deteriorated over time until it naturally ruptured approximately 8 weeks after MRFE treatment. Previous studies have shown that rupture or surgical transectioning of the CCL or meniscectomy in experimental animals favors the development of OA21–25. This MRFE model avoids surgical sectioning of the CCL. The concentrations of COL2-3/4C long neoepitope and PG chondroitin-sulfate epitopes 3B3(–) and 846 were measured in synovial fluid collected at different time points before and after CCL rupture.
Materials and methods
ANIMAL MODEL
Eighteen mature mixed-breed dogs were used with the approval of the institutional Animal Use and Care Committee. All animals underwent bilateral stifle arthroscopy during which MRFE (70°C, 25 W, ORA-50, Oratec Interventions, Inc. Menlo Park, CA) was applied to one randomly selected CCL while the contralateral stifle was operated identically except that no energy was applied to the CCL (sham treatment). Animal activity was unrestricted after surgery. Rupture of the CCL in each treated limb was detected by a sudden onset of non-weight-bearing lameness, significant joint effusion, and a positive drawer sign in the affected joint. Joint effusion was not detectable prior to CCL rupture26. Complete dissolution of all treated CCLs was confirmed at necropsy, and joints had osteoarthritic changes characteristic of the canine CCL transection model of OA21–23. Sham treatment joints were free of pathologic changes and the CCL and caudal cruciate ligament were intact. As part of an unrelated study, stifle radiographs (caudocranial and lateromedial) were taken pre-operatively and 4 weeks prior to rupture, and 4, 8, and 16 weeks following rupture26. Radiographs were evaluated for standard signs of OA including the presence of osteophytes, enthesiophytes, subchondral bone sclerosis, and, following CCL rupture in one limb, cranial displacement of the tibia relative to the femur. As mentioned previously, the dogs were part of an unrelated study, which required sacrifice at different time points, hence the number of animals decreased over the duration of the study26.
SAMPLE COLLECTION AND PREPARATION
Synovial fluid was aseptically collected (0.2–2.0 ml) from each stifle. Specifically, 36 samples were collected from 18 dogs at time 0 (8 weeks prior to CCL rupture), 4 weeks prior to rupture, and 4 and 8 weeks following rupture, and 24 samples were collected from 12 dogs at 16 weeks following rupture. The samples were centrifuged at 10,000 rpm for 10 min, and the supernatant was aliquoted and stored at −80°C until analysis. For COL2-3/4C long and 846 assays, proteinase inhibitors were added to the samples which included 1 mM EDTA, 1 mM PMSF (in isopropanol), 1 mM iodoacetarnide and 5 μg/ml pepstatin A (in 100% ethanol) in 50 mM sodium acetate buffer, pH 5.0. Prior to assay enzymatic digestion of hyaluronic acid was performed at 37° overnight with 0.4 TRU/ml of Streptomyces hyaluronidase (Seikagaku Inc., Japan). For the 3B3(–) assay, undigested samples of synovial fluid were assayed after making appropriate dilutions.
ELISAS
Immulon II microplates (Dynamics) were used for CQL2-3/4C long and 3B3(–) assays and duplicate samples were used for each.
COL2-3/4C long neoepitope
Our previous data have shown that this monoclonal antibody, which is specific for collagenase-cleaved type II collagen, reacts with dog type II collagen cleaved by collagenase 1 (MMP-1) (M. Ionescu and A. R. Poole, unpublished). Peptide 287-KLH diluted in PBS, pH 7.2 was used to coat Immulon II microplates at 5 ng/well (50 μl/well). The plates were incubated overnight at 4°C. After washing three times with PBS containing 0.1% Tween 20 with a plate washer (ELP-40, BIO-TEK Instruments), the plates were blocked with 100 μl of 1% BSA in PBS, pH 7.2 for at least 30 min at room temperature, followed by washing three times as above. A standard curve was made with peptide 287 ranging from 1000 ng–1.3ng/ml diluted with PBS buffer, pH 7.2, containing 1% BSA. Fifty μl of synovial fluid samples/standard was added to a round bottom polypropylene plate, then 50 μl of COL2-3/4 long monoclonal antibody diluted at 1:120,000 in PBS containing 1% BSA and 0.1% Tween20, was added to the plate. After 1-h incubation at 37°C, 50μl of the antigen-antibody mixture was transferred to the coated Immulon II plate and incubated for 30 min at 4°C. The plate was washed three times as before. Secondary antibody, Goat anti-Mouse IgG, M, A (H+L) Alkaline Phosphatase Conjugate (Zymed) diluted at 1:5,000 in PBS containing 1% BSA and 0.1% Tween20, was added to the plate at 50 μl per well. After 1-h incubation at 37°C and washing the plate as above, 50 μl of substrate (ELISA Amplification System, GIBCO BRL) was added to the plate and incubated for 15 min at room temperature. Fifty μl of amplifier (ELISA Amplification System, GIBCO BRL) was then added for further 15-min incubation. The reaction was stopped by adding 50 μl of 0.3 M H2SO4. OD was obtained by reading the plate at 490 nm on an ELISA plate reader (EL 312e, Bio-Kinetics Microplate Reader, BIO-TEK Instruments).
3B3(–) epitope
A competitive ELISA method similar to that previously described27 was used to determine the concentrations of 3B3(–) epitope in synovial fluid. Porcine aggrecan core protein was kindly supplied by Dr Michael T. Bayliss, Royal Veterinary College, London. Monoclonal antibody 3B3 was purchased from Seikagaku, Japan.
846 epitope
This was determined by radioimmunoassay, essentially as previously described19. Bovine fetal PG was labeled with 125I as the tracer, and iodination was completed by using a standard procedure28. Bovine fetal aggrecan was used as standard. All the samples were assayed in triplicate.
Recoveries from each neoepitope and epitope were determined by adding the standards to pooled samples.
STATISTICS
Unpaired Student’s t-tests were used to compare neoepitope or epitope concentrations between treated and sham operated joints. ANOVA was used to evaluate the effect of time and groups. When ANOVA revealed significant differences over time, a post-hoc t-test (Scheffe’s procedure) was performed to analyse these differences. Simple linear regression analysis was performed to correlate concentrations of markers and time points and to correlate concentrations of 3B3(–) and COL2-3/4C long. Differences were considered significant at P<0.05.
Results
CCL RUPTURE
Rupture of treated CCL’s was detected in 14 dogs by sudden onset of non-weight bearing lameness, joint effusion, and a positive drawer sign with joint manipulation. The exact dates of rupture were not observed in four dogs. The values from these animals were not used to calculate the mean rupture date. Treated CCLs ruptured approximately 8 weeks after treatment (55±1.6; 48–66 days) (Mean±S.E.M.; range).
RADIOGRAPHIC CHANGES
The radiographic changes that occurred in joints with MRFE treated CCLs and sham treated CCLs have been reported previously26. Pre-operative and 4 week pre-rupture radiographs did not have any significant changes. Joints with sham treated CCLs did not have any significant radiographic changes for the duration of the study. Four weeks after CCL rupture, radiographs of stifles with treated CCLs demonstrated significant joint effusion and some degree of cranial displacement of the tibia relative to the femur on the lateromedial view, both of which remained present for the remainder of the study. The majority of the joints with treated CCLs (14/18) had very early osteophyte formation on the proximal trochlear ridges within the femoropatellar joint. Radiographs performed on limbs with treated CCLs 8 weeks post-rupture showed varying degrees of osteoarthritic changes on the tibial intercondylar eminences and femoral trochlear ridges. By 16 weeks after CCL rupture, significant radiographic changes consisted of osteophytosis of the tibial articular surface and the trochlear ridges of the femur and slight subchondral bone sclerosis of both the tibia and femur. Radiographic changes in the 8 and 16 week post-rupture radiographs varied in severity, but all joints had some degree of the changes listed.
COL2-3/4C LONG NEOEPITOPE
There were no differences between treated and sham groups at 4 and 8 weeks prior to CCL rupture (Fig. 1). However, after CCL rupture the neoepitope concentrations were significantly increased: 1.7 fold at 4 weeks (P<0.0001), 2.2 fold at 8 weeks (P<0.0001) and 1.7 fold at 16 weeks (P<0.02), compared with sham treated joints. There were no differences over time between the sham treated joints. Within the MRFE treated groups, COL2-3/4C long neoepitope concentrations were significantly higher at 4 weeks and 8 weeks after rupture than at 4 and 3 weeks prior to CCL rupture.
Fig. 1.
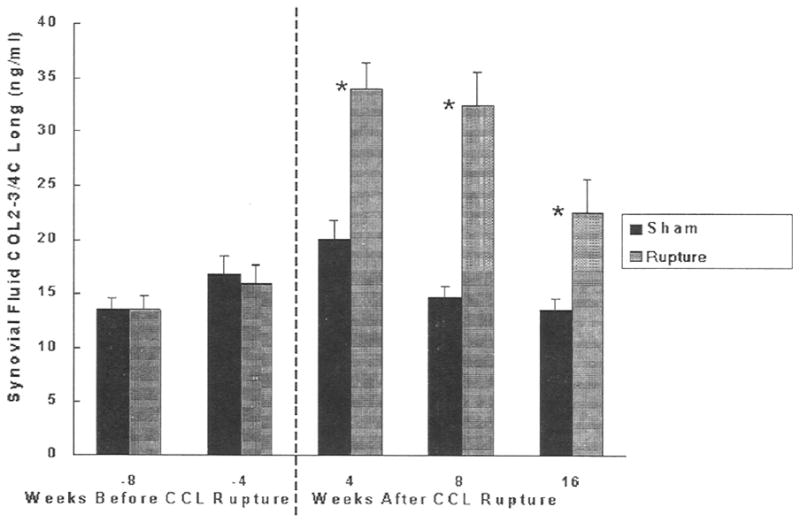
Concentrations of COL2-3/4C long neoepitope in dog synovial fluid in CCL ruptured joint and sham-operated control. Means ± standard deviations are shown. *Significantly different (P<0.05).
3B3(–) EPITOPE
There were no differences between MRFE treated and sham groups at 4 and 8 weeks prior to CCL rupture (Fig. 2). 3B3(–) epitope concentrations were significantly increased in all the CCL ruptured groups compared to the sham treated joints. They were 1.7 fold higher at 4 weeks (P<0.0001), 2.9 fold higher at 8 weeks (P<0.0001) and 2.4 fold higher at 16 weeks (P<0.0001) following CCL rupture compared to the sham operated joints. Within the MRFE treated group, 3B3(–) epitope concentrations at 4, 8, and 16 weeks following rupture were higher than those 8 weeks before rupture, Moreover, concentrations at 4 and 8 weeks following rupture were higher than those at 4 weeks prior to rupture. Significant differences among some time points within the sham treated group were also identified. Concentrations at 4 weeks prior to CCL rupture were higher than 8 and 16 weeks after CCL rupture. An increase in 3B3(–) concentrations was correlated significantly with increasing COL2-3/4C long concentrations (P<0.0001, r2=0.23) (Fig. 3).
Fig. 2.
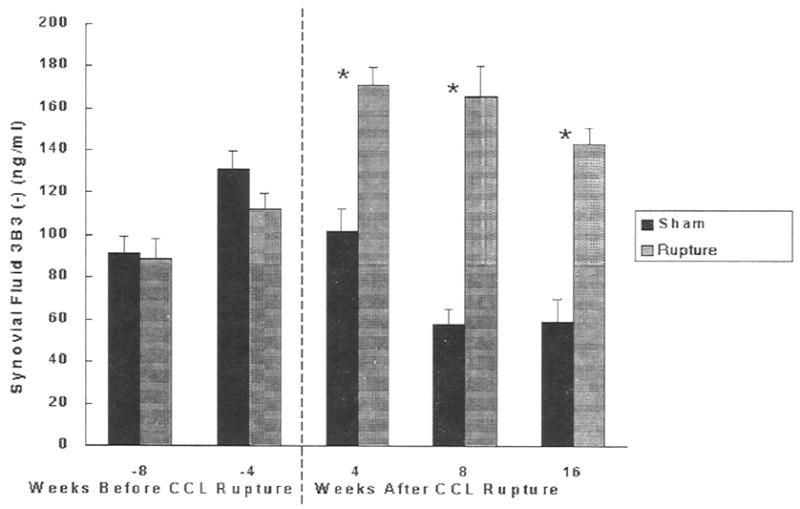
Concentrations of PG chondroitin sulfate 3B3(–) epitope in dog synovial fluid in CCL ruptured joint and sham-operated control. Means ± standard deviations are shown. *Significantly different (P<0.05).
Fig. 3.
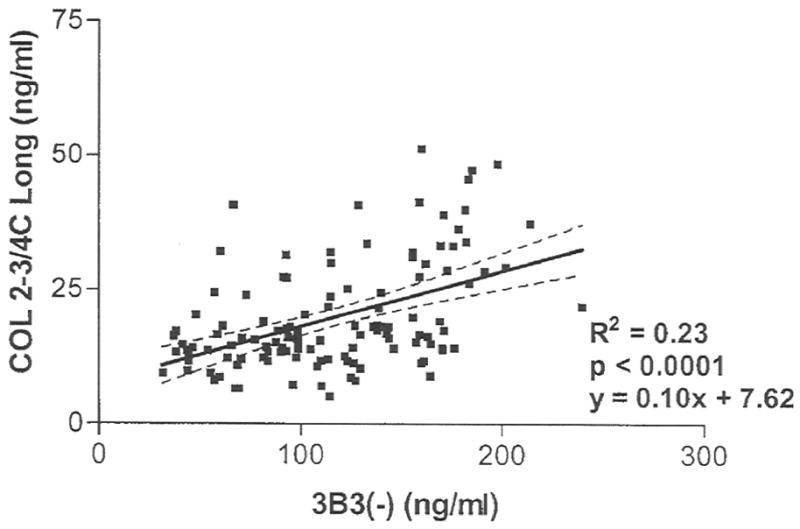
Linear regression of synovial fluid COL2-3/4C long neoepitope to synovial fluid 3B3(–) epitope concentrations.
846 EPITOPE
The concentrations of 846 epitope showed a trend for an increase, which was not significant, in the MRFE treated group, starting at 4 weeks after CCL rupture and remained higher until 16 weeks after CCL rupture (Fig. 4). Regression analysis showed a weak but significant correlation (r2=0.35, P<0.01) between 846 epitope concentration and duration of CCL rupture. The concentrations initially decreased and then increased at later time points. There were no differences within the sham operated group over time.
Fig. 4.
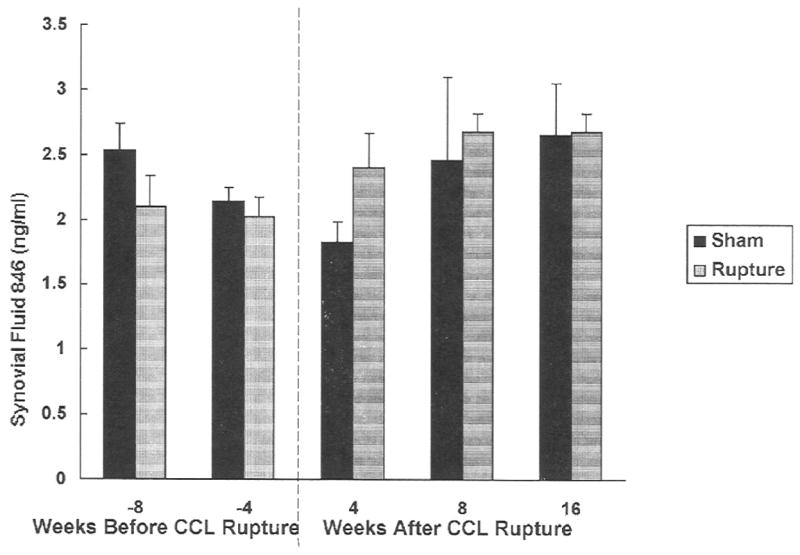
Concentrations of PG chondroitin sulfate 846 epitope in dog synovial fluid in CCL ruptured joint and sham-operated control. Means±standard deviations are shown.
Average recovery rate for COL2-3/4C long assay was 112±4.0%; 99±3.0% for 3B3(–) and 91.5±3.5% for 846 assays.
Discussion
This study examined the potential for biochemical markers to detect OA in a novel model of canine CCL disruption. The use of MRFE to cause the degeneration and rupture of the canine CCL approximately 8 weeks after surgery allowed resolution of many of the changes caused by surgical manipulation of the joints. Hence, concentrations of COL2-3/4C long, 3B3(–), and 846 could be evaluated as they changed relative to the changes in joint health as a result of joint instability. The goal of the study was to evaluate the markers for their potential to detect the earliest signs of articular surface disruption even before changes were evident in alt joints radiographically. Using this novel model of canine CCL disruption, there was increased degradation of type II collagen and changes in aggrecan turnover early following induction of joint instability. The results suggest that in the dog, the COL2-3/4C long neoepitope and the 3B3(–) epitope are useful markers for the detection of early changes in cartilage metabolism during the development of OA.
Biochemical markers may be useful for early diagnosis, determining contributing factors, and monitoring disease progression and response to therapy during the development of OA. Cartilage degradation and synthesis can now be detected in vitro by using antibodies29. The collagenase generated type II collagen cleavage can be detected and measured16. Synthesis of type II procollagen can be detected and measured by measuring the C-propeptide of the molecule30. Agreccan epitopes of keratan sulfate31, 3B3(–)32 and 84620 can also be detected with antibodies. In this study, we examined type II collagen neoepitope COL2-3/4C long, and PG epitopes 3B3(–) and 846 as biochemical markers of cartilage turnover. The two major macromolecules of articular cartilage extracellular matrix are type II collagen and the proteoglycan aggrecan. The neoepitope COL2-3/4C long generated by collagenase cleavage of type II collagen is a logical choice since collagenase activity is considered to play a significant role in the cleavage of type II collagen and it is elevated in OA16. However, the chondroitin sulfate epitopes 3B3(–) and 846 are present in developing tissues, disappear in the adult and reappear in osteoarthritic cartilage2,14. The latter changes might represent an attempt to repair the altered microenvironment2,14. We measured the concentrations of these three markers in the synovial fluid of dogs with joint instability that would favor development of OA to determine changes in their levels at different time points in the disease progression.
Creation of joint instability by transection of the CCL and meniscectomy has been clearly shown to result in OA in the dog22–25. We applied MRFE arthroscopicalty to one CCL in each dog, without performing an arthrotomy or transecting ligaments, thus resulting in an accelerated but naturally occurring CCL rupture with minimal surgical trauma. Synovial fluid concentrations of COL2-3/4C long neoepitope and 3B3(–) epitope were unchanged at 4 weeks after MRFE treatment (4 weeks before rupture). Significant increases in the COL2-3/4C long neoepitope and 3B3(–) epitope concentrations were observed at 4 weeks after CCL rupture and the concentrations remained significantly higher than the sham operated controls for at least 16 weeks following rupture.
The elevation of COL2-3/4C long neoepitope in the synovial fluid from the current study reflects the collagenase activity that leads to excessive degradation of collagen (A.R. Poole, L. Dahlberg, M. lonescu and R.C. Billinghurst unpublished). Degradation of cartilage matrix involves the extracellular cleavage of matrix molecules. Collagenases MMP-1, MMP-8, and MMP-13 are the enzymes currently known to cleave the cartilage matrix collagen16. These enzymes mainly originate in synovial cells and Chondrocytes33 In the early stages of OA, cartilage attempts to repair the tissue damage. Chondrocytes may proliferate and secrete more degradative enzymes, modulated by certain cytokines, leading to excessive degradation of cartilage matrix molecules. We did not measure the levels of collagenases in this study. However, increased denaturation of the triple helix of type II collagen, in which collagenases are involved, has been reported to occur in human articular cartilage during OA development7,17. Another study has shown that the immunoreactivities for collagenase-1 and collagenase-3 were increased dramatically in the early phases of experimentally induced canine OA34. Similarly, our study showed significant elevations in the synovial fluid concentrations of COL2-3/4C long neoepitope 4, 8, and 16 weeks after CCL rupture compared to controls. This reveals an abnormal degradation of type II collagen in the early phases of canine OA.
The 3B3(–) changes we noted are similar to the findings of a previous study using an acute canine cranial cruciate ligament transection model in which there were significant elevations of 3B3(–) levels in synovial fluid from CCL transected joints, compared to the controls33. 3B3(–) epitope levels in synovial fluid are also elevated following post-traumatic OA caused by surgical damage to the menisci in dogs. The levels of 3B3(–) epitope peaked at 4 weeks after meniscectomy, and remained above the baseline at 12 weeks25. This indicates changes in aggrecan turnover in early stages of OA indicative of cartilage degradation. Interestingly, concentrations of 3B3(–) epitope and COL2-3/4C long neoepitope both peaked at 8 weeks after CCL rupture (2.9 fold for 3B3(–), and 2.2 fold for COL2-3/4C). In addition, there was a significant positive correlation between the 3B3(–) epitope and COL2-3/4C long neoepitope (P<0.0001, r2=0.23). This may best be explained as increased damage to cartilage type II collagen that is combined with cartilage aggrecan turnover. In such cases, chondrocytes exhibit increased proliferation and there is an increase in matrix synthesis36. Degradation and synthesis of type II collagen and aggrecan occur together with the development of OA. The concentrations of 3B3(–) epitope from the sham treated group showed a slight increase after MRFE treatment of the contralateral CCL. The reasons for this remain to be established.
The concentration of the 836 epitope showed an increasing trend at 4 weeks after CCL rupture in the MRFE treated group, but it was not statistically different from sham operated controls. This epitope is increased in OA cartilage4 and in human knee joints following injury20. It is at its highest in human knee OA where synovial fluid content is markedly increased5,20. In rabbit OA induced by cranial cruciate ligament transection and medial meniscectomy, 846 epitope is also elevated at 12 weeks (A. R. Poole, L. Killar et al., unpublished). Thus, this difference may be peculiar to the dog or the model. Further study is required to demonstrate whether an elevation of 846 epitope in aggrecan extracted from fetal canine cartilage is present when compared with aggrecan from normal adult canine cartilage.
We are presenting the information in this paper as a potential technique by which joint disease can be assessed with greater sensitivity and specificity than standard imaging modalities. Our purpose was to establish methods by which disease progression and therapeutic effects can be assessed both clinically and for research purposes. With this thought in mind, we feel that the neoepitope/epitope concentrations in the joint fluid are more significant than their absolute concentrations. This is so because we are assuming that though the disease of OA is progressive, changes in the concentrations at any given time are so slight that they may be considered to be at steady-state concentrations.
In summary, using a novel model of canine CCL disruption, COL2-3/4C long and 3B3(–) were found to be useful indicators of early changes in cartilage metabolism which predicted the onset of radiographic changes characteristic of OA. These markers may prove very useful in research studies seeking to prevent or alleviate the manifestations of OA prior to irreversible changes. Further studies are required to determine potential clinical applications of the markers, but it is possible that that they may be used in cases where joint pain occurs before radiographic evidence of OA.
Acknowledgments
Financial support: National Institutes of Health, Oratec Interventions, Inc., Menlo Park, CA (to MDM), and National Institutes of Health, Canadian Institutes of Health Research, Canadian Arthritis Network and Shriners Hospitals for Children (to ARP).
References
- 1.Rorvik AM, Grondahl AM. Markers of osteoarthritis: A review of the literature. Vet Surg. 1995;24:255–62. doi: 10.1111/j.1532-950x.1995.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 2.Caterson B, Hughes CE, Roughley P, Mort JS. Anabolic and catabolic markers of proteoglycan metabolism in osteoarthritis. Acta Orthop Scand. 1995;66(suppl 266):121–4. [PubMed] [Google Scholar]
- 3.Caterson B, Hughes CE, Johnstone B, Mort JS. Immunological markers of cartilage proteoglycan metabolism in animal and human osteoarthritis. In: Kuettner KE, Schleyerbach R, Peyron JG, Hascall VC, editors. Articular Cartilage and Osteoarthritis. New York: Raven Press; 1992. pp. 415–27. [Google Scholar]
- 4.Rizkalla G, Reiner A, Bogoch E, Poole AR. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. J Clin Invest. 1992;90:2268–77. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole AR, lonescu M, Swan A, Dieppe PA. Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan. J Clin Invest. 1994;94:25–33. doi: 10.1172/JCI117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisbie DD, Ray CS, lonescu M, Poole AR, Chapman PL, Mcllwraith CW. Measurement of synovial fluid and serum concentrations of the 846 epitope of chondroitin sulfate and of carboxy propeptides of type II procollagen for diagnosis of osteochondral fragmentation in horses. Am J Vet Res. 1999;60:306–9. [PubMed] [Google Scholar]
- 7.Dodge GR, Poole AR. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989;83:647–61. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazell PK, Dent C, Fairclough JA, Bayliss MT, Hardingham TE. Changes in glycosaminoglycan epitope levels in knee joint fluid following injury. Arthritis Rheum. 1995;38:953–9. doi: 10.1002/art.1780380711. [DOI] [PubMed] [Google Scholar]
- 9.Ratcliffe A, Shurety W, Caterson B. The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum. 1993;36:543–51. doi: 10.1002/art.1780360416. [DOI] [PubMed] [Google Scholar]
- 10.Carlson CS, Loeser RF, Johnstone B, Tulli HM, Dobson DB, Caterson B. Osteoarthritis in cynomolgus macaques. II. Detection of modulated proteoglycan epitopes in cartilage and synovial fluid. J Orthop Res. 1995;13:399–409. doi: 10.1002/jor.1100130314. [DOI] [PubMed] [Google Scholar]
- 11.Poole AR. Immunology of cartilage. In: Moskowitz RW, Howell DS, Goldberg VM, Mankin HJ, editors. Osteoarthritis Diagnosis and Medical/Surgical Management. 2. Philadelphia: W. B. Saunders Company; 1992. pp. 155–89. [Google Scholar]
- 12.Kempson G. The mechanical properties of articular cartilage. In: Sokoloff L, editor. The Joints and Synovial Fluid. II. New York: Academic Press; 1980. pp. 177–238. [Google Scholar]
- 13.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353–63. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 14.Poole AR. Cartilage in health and disease. In: Koopman WJ, editor. Arthritis and Allied Conditions: A Textbook of Rheumatology. 13. Baltimore: Williams and Wilkins; 1997. pp. 255–308. [Google Scholar]
- 15.Vankemmelbeke M, Dekeyser PM, Hollander AP, Buttle DJ, Demeester J. Characterization of helical cleavages in type II collagen generated by matrixins. Biochem J. 1998;330:633–40. doi: 10.1042/bj3300633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billinghurst RC, Dahlberg L, lonescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenase in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–45. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–32. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plaas AHK, West LA, Wong-Palms S, Nelson FRT. Glycosaminoglycan sulfate in human osteoarthritis. J Biol Chem. 1998;273:12642–9. doi: 10.1074/jbc.273.20.12642. [DOI] [PubMed] [Google Scholar]
- 19.Glant TT, Mikecz K, Roughley PJ, Buzas E, Poole AR. Age-related changes protein-related epitopes of human articular-cartilage proteoglycans. Biochem J. 1986;236:71–5. doi: 10.1042/bj2360071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohmander LS, lonescu M, Jugessur H, Poole AR. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum. 1999;42:534–44. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Matyas JR, Ehlers PF, Huang D, Adams ME. The early molecular natural history of experimental osteoarthritis. I. Progressive discoordinate expression of aggrecan and type II procollagen messenger RNA in the articular cartilage of adult animals. Arthritis Rheum. 1999;42:993–1002. doi: 10.1002/1529-0131(199905)42:5<993::AID-ANR19>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Visco DM, Johnstone B, Hill MA, Jolly GA, Caterson B. Immunohistochemical analysis of 3-B-3(-) and 7-D-4 epitope expression in canine osteoarthritis. Arthritis Rheum. 1993;36:1718–25. doi: 10.1002/art.1780361211. [DOI] [PubMed] [Google Scholar]
- 23.Hay CW, Chu Q, Budsberg SC, Clayton MK, Johnson KA. Synovial fluid interleukin 6, tumor necrosis factor, and nitric oxide values in dogs with osteoarthritis secondary to cranial cruciate ligament rupture. Am J Vet Res. 1997;58:1027–32. [PubMed] [Google Scholar]
- 24.Lanzer WL, Komenda G. Changes in articular cartilage after meniscectomy. Clin Orthop Relat Res. 1990;252:41–8. [PubMed] [Google Scholar]
- 25.Lindhorst E, Vail TP, Guilak F, Wang H, Setton LA, Vilim V, Kraus VB. Longitudinal characterization of synovial fluid biomarkers in the canine meniscectomy model of osteoarthritis. J Orthop Res. 2000;18:269–80. doi: 10.1002/jor.1100180216. [DOI] [PubMed] [Google Scholar]
- 26.Lopez MJ, Kunz D, Vanderby R, Jr, Heisey D, Bogdanske J, Markel MDM. A comparison of joint mechanics between anterior cruciate intact and deficient stifles: a new canine model of anterior cruciate ligament disruption. doi: 10.1016/S0736-0266(02)00132-8. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bautch JC, Clayton MK, Chu Q, Johnson KA. Synovial fluid chondroitin sulphate epitopes 3B3 and 7D4, and glycosaminoglycan in human knee osteoarthritis after exercise. Ann Rheum Dis. 2000;59:887–91. doi: 10.1136/ard.59.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller WT, Smith JFB. Protein iodination using Iodo-Gen. Int J Appl Radiat Isot. 1983;34:639–41. doi: 10.1016/0020-708x(83)90068-6. [DOI] [PubMed] [Google Scholar]
- 29.Poole AR. An introduction to the pathophysiology of osteoarthritis. Front Biosci. 1999;4:d662–70. doi: 10.2741/poole. [DOI] [PubMed] [Google Scholar]
- 30.Nelson F, Dahlberg L, Reiner A, Pidoux I, Fraser G, Brooks E, et al. The synthesis of type II procollagen is markedly increased in osteoarthritic cartilage in vivo. J Clin Invest. 1998;102:2115–25. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thonar EJ-MA, Lenz ME, Klintworth GK. Quantitation of keratan sulfate in blood as marker of cartilage metabolism. Arthritis Rheum. 1985;28:1367–76. doi: 10.1002/art.1780281209. [DOI] [PubMed] [Google Scholar]
- 32.Johnson KA, Hart RC, Chu Q, Kochevar D, Hulse DA. Concentrations of chondroitin sulfate epitope 3B3 and 7D4 in synovial fluid after intra-articular and extra capsular reconstruction of the cranial cruciate ligament in dogs. Am J Vet Res. 2001;62:581–7. doi: 10.2460/ajvr.2001.62.581. [DOI] [PubMed] [Google Scholar]
- 33.Smith RL. Degradative enzymes in osteoarthritis. Frontiers in Bioscience. 1999;4:d704–12. doi: 10.2741/a388. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes JC, Martel-Pelletier J, Lascau-Coman V, Moldovan F, Jovanovic D, Raynauld JP, Pelletier JP. Collagenase-1 and collagenase-3 synthesis in normal and early experimental osteoarthritic canine cartilage: an immunohistochemical study. J Rheumatol. 1998;25:1585–94. [PubMed] [Google Scholar]


