Figure 1.
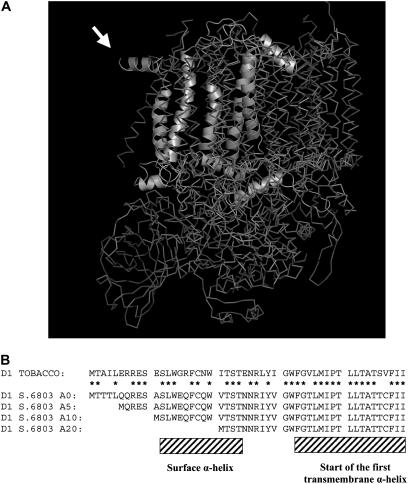
Cyanobacterial PSII Structure and the N Terminus of D1 in the Constructed N-Terminal Truncation Mutants of Synechocystis sp PCC 6803.
(A) Crystal structure of PSII from Thermosynechococcus elongatus showing the N-terminal region of the D1 subunit exposed at the periphery of the complex. The D1 polypeptide chain is shown only in one of the PSII monomers in the dimer. This image is modeled from the coordinates determined by Ferreira et al. (2004). Note that the first nine residues of D1 cannot be resolved in the structure. Amino acid residues 11 to 20, forming the parallel helix protruding from the structure, are indicated by the arrow.
(B) Comparison of the decoded N-terminal sequences (residues 1 to 50) of D1 from Synechocystis sp PCC 6803 and tobacco (Nicotiana tabacum). Shown are the positions of the surface α-helix and the beginning of the first transmembrane α-helix plus the amino acid residues deleted in the A5, A10, and A20 mutants. Stars indicate residues conserved in chloroplast and cyanobacterial sequences.