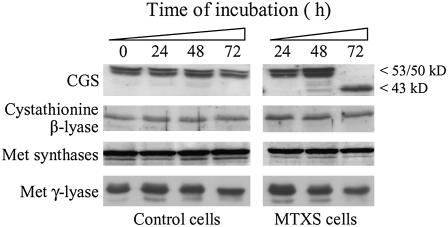
Figure 4.
Expression of the enzymes involved in Met synthesis and catabolism in folate-sufficient and folate-deficient cells. Soluble proteins (40 μg per lane) from Arabidopsis cells grown in standard medium (control cells) or exposed to 100 μm MTX and 100 μm sulfanilamide (MTXS cells) were analyzed by western blot using antibodies raised against CGS, cystathionine β-lyase, Met synthases, and Met γ-lyase from Arabidopsis. The 53- and 50-kD polypeptides detected with the CGS serum are characteristic of the mature enzyme, the one at 43 kD being observed only in cells starved for folates for 72 h. Quantitation of CGS polypeptides using chemiluminescence detection reagents and a Typhoon 9400 scanner indicated that the amount of CGS protein was increased by 2-fold between 24 and 48 h of treatment and was maintained constant between 48 and 72 h (titration experiments using recombinant proteins indicated that the signal obtained with the 43-kD polypeptide of CGS was reduced by 30%–40% as compared with the signal measured for the mature protein). The antibodies against Met synthase cross-react with both the cytosolic (top band) and chloroplastic (bottom band) isoforms of the enzyme (Ravanel et al., 2004a).