Abstract
Crown gall tumors induced by Agrobacterium tumefaciens represent a sink that has to be provided with nutrients and water by the host plant. The lack of an intact epidermis or cuticle results in uncontrolled loss of water. However, neither the tumor nor the host plant displays wilting. This phenomenon points to drought adaptation in both tumors and the crown gall host plant. To understand the underlying molecular mechanisms of protection against desiccation the gene expression pattern of Arabidopsis (Arabidopsis thaliana) tumors was integrated with the profile of stress metabolites: Arabidopsis tumors accumulated high amounts of abscisic acid (ABA), the ethylene precursor aminocyclopropyl carboxylic acid, osmoprotectants, and form a suberized periderm-like protective layer. Suberization of the outer tumor cell layers most likely is mediated by ABA since external application of ABA induced suberization of Arabidopsis roots. However, the expression level of the classical marker genes, known to respond to drought stress and/or ABA, was lower in tumors. Instead another set of drought and/or ABA-inducible genes was more highly transcribed. Elevated transcription of several ABA-dependent aquaporin genes might indicate that ABA controls the water balance of the tumor. The retarded tumor growth on abi and aba mutant plants underlined the importance of a tumor-specific ABA signaling pathway. Taken together, we propose that ABA is an important signal for protection of tumors against desiccation and thus supports tumor development.
Infection of plants by Agrobacterium tumefaciens can induce plant tumors, also referred to as crown galls, which develop upon integration of an Agrobacterium-derived T-DNA into the host plant genome (pMP90; Thomashow et al., 1980). Expression of the T-DNA-encoded oncogenes in turn promote biosynthesis of auxin and cytokinin, resulting in non-plant-controlled production of these phytohormones. This initiates cell proliferation as well as differentiation of a vascular network, which will connect the tumor to vascular bundles of the host plant and thus support the tumor with nutrients and water (Kado, 1984; Aloni et al., 1995; Mistrik et al., 2000; Ullrich and Aloni, 2000).
Tumors display a ruptured surface that is devoid of a cuticle covered epidermal cell layer; as a result tumors exhibit an increased water loss (Schurr et al., 1996). Usually, this is prevented by specialized cell types that contain substantial amounts of aliphatic polymers like cutin or suberin: Cutin is a major component of the cuticle that covers all aerial primary organs and forms the interface between plant and atmosphere. In contrast, the deposition of suberin in plants is highly variable: Suberin is constitutively present in several underground tissues, including root endodermal and hypodermal cell walls. In addition, it is present in the periderm that forms the outer tissue of stems during the secondary growth stage (Kolattukudy, 1981; Zeier and Schreiber, 1998; Nawrath, 2002; Franke et al., 2005). Suberin is also deposited in response to abscisic acid (ABA; a phytohormone) wounding and upon pathogen attack to establish a water-impermeable barrier (Soliday et al., 1978; Cottle and Kolattukudy, 1982; Vogt et al., 1983).
Protection against drought can also be achieved by a decrease in cellular osmotic potential due to synthesis and/or accumulation of osmoprotective compounds (see Mahajan and Tuteja, 2005 and refs. therein). These small, nontoxic compounds (e.g. several amino acids, like Pro and Glu, as well as sugars and inorganic ions) can stabilize proteins and cellular structures. Additionally, this accumulation of osmoprotectants increases the osmotic pressure of the cell resulting in water uptake (Yancey et al., 1982).
Adaptation to water stress results from an alteration in gene expression by up-regulation of the major ABA- and/or stress-responsive genes, like RD (response to dehydration), COR (cold responsive), LEA (late embryogenesis abundant)/dehydrin-like, and aquaporin genes (Seki et al., 2001). Their products activate chaperones to protect cellular proteins from degradation, activate proteinases to remove damaged proteins, and aquaporins are likely to control the water status of plant cells (Seki et al., 2001; Siefritz et al., 2002; Zhu et al., 2005). In plants, drought protection is mainly triggered by ABA, the key phytohormone involved in response to abiotic stress (Mahajan and Tuteja, 2005). Genes encoding enzymes involved in ABA biosynthesis are up-regulated in response to drought. This results in a rapid, feed-forward accumulation of ABA and induction of adaptive mechanisms (Zeevaart and Creelman, 1988). Arabidopsis (Arabidopsis thaliana) mutants affected in ABA signaling identified this pathway as essential for desiccation tolerance (Koornneef et al., 1984).
Here we have investigated the role of drought protective mechanisms during tumor development of A. tumefaciens (strain C58) induced crown galls. In contrast to previous studies (e.g. Mistrik et al., 2000; Veselov et al., 2003; Wächter et al., 2003) we took advantage of the genetic model plant Arabidopsis to dissect the role of ABA signaling in drought protection during crown gall development. We applied microarray techniques to investigate expression of the well-known marker genes for drought stress and/or ABA. On a functional level, we took advantage of Arabidopsis mutants impaired in ABA biosynthesis or signaling to investigate its role in tumor development.
RESULTS
ABA Accumulation in Tumors Is Not Reflected on the Level of Gene Expression
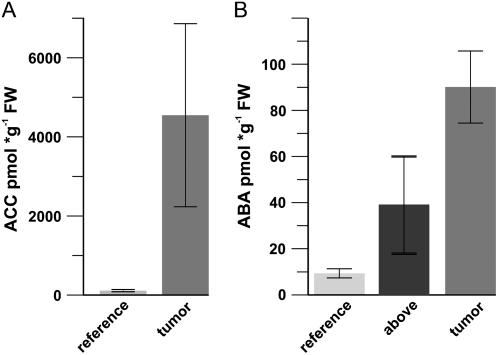
In the search for triggers that might promote drought resistance of tumors we analyzed contents of the stress hormones ABA and aminocyclopropyl carboxylic acid (ACC), the latter of which is a precursor of the gaseous ethylene and can induce ABA biosynthesis (Hansen and Grossmann, 2000). ACC was increased 45-fold compared to reference stalks of uninfected plants (Fig. 1A). To investigate the expression pattern of genes involved in biosynthesis of this phytohormone precursor we have applied Affymetrix microarrays (approximately 22 K) that nearly represent the complete genome of Arabidopsis (Deeken et al., 2006). Genes encoding the ACC synthases (ACS8 and ACS2) and ACC oxidase (ACO1) revealed increased expression in Arabidopsis tumors, consistent with elevated ACC contents (Supplemental Fig. S1A).
Figure 1.
Tumors accumulate ACC and ABA. A, ACC content in tumors and inflorescence stalk tissue (reference). B, ABA content in tumors, inflorescence stalk (reference), and in the stalk above the tumors (above). For each of the three experiments, tissue from at least 10 plants was analyzed. Bars represent mean values (±sd) of three independent experiments.
Tumors and inflorescence stalks above the tumor exhibited a significant increase in ABA content compared to reference stalks of uninfected plants (around a 10-fold increase in tumor and 4-fold in the stalk above the tumor, respectively; Fig. 1B). However, this increase in ABA content is not reflected by the expression level of genes encoding enzymes of ABA biosynthesis or degradation (for review, see Nambara and Marion-Poll, 2005): Zeaxanthin epoxidase (AtZEP) and 9-cis-epoxycarotenoid dioxygenases (AtNCED1 and AtNCED4) were either decreased or unchanged (see AtNCED2, AtNCED3, AtNCED5, and AtNCED6 in Supplemental Fig. S1B). ABA2 and AAO3, encoding enzymes catalyzing the conversion of xanthoxal to abscisic aldehyde, or abscisic aldehyde oxidase, respectively, were not up-regulated (Supplemental Fig. S1B). The reliability of the microarray expression data for genes of the ABA biosynthesis pathway has been confirmed by real-time reverse transcription PCR (data not shown).
We found no evidence for ABA accumulation as a consequence of impaired degradation or an increase in release from conjugated forms, since gene expression of members of the CYP70A7 gene family (CYP70A7-1 to CYP70A7-4; Supplemental Fig. S1B), key enzymes of ABA degradation, remained unchanged in the tumor. AtBG1, encoding a β-glucosidase, hydrolyzing Glc-conjugated inactive ABA to free ABA (Bindschedler et al., 2006), was 4-fold down-regulated in tumors. This result is in agreement with the 2-fold higher accumulation of ABA-Glc esters in tumors of hydroponically cultivated Arabidopsis plants compared to control tissues (data not shown).
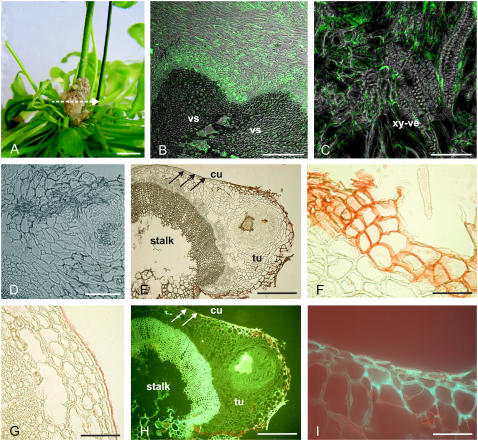
These data suggest that the elevated content of ABA in tumors is not autonomously synthesized in this tissue but might rather be translocated into the tumor by the host plant. Therefore, the distribution of ABA in tumor cross sections (Fig. 2A) was visualized using antibodies against ABA following the method of Schraut et al. (2004). Strong signals were observed near vascular tissues that connect the tumor with the inflorescence stalk of the host (Fig. 2B). Cells adjacent to xylem vessels exhibited strong signals, which were restricted to the cytoplasmatic layer (Fig. 2C). The specific distribution of ABA at the interface between tumor and host plant supports the idea of ABA being translocated into tumors.
Figure 2.
Localization of suberin and ABA in tumors. A, Four-week-old tumor, induced by A. tumefaciens (strain C58) at an inflorescence stalk of Arabidopsis. The arrow indicates the section plane of the cross sections shown in B to I. B to D, ABA immunolocalization in tumor cross sections using a primary mouse hybridoma monoclonal antibody against ABA and a secondary antibody labeled with the green Alexa 488 chromophore. Images were taken with a confocal laser scanning microscope and show overlays of images taken in a confocal and differential interference contrast mode. B, Fluorescence signals were strongest around vascular tissue (vascular, vs) of the tumor/host inflorescence stalk interface. C, ABA immunofluorescence at cellular resolution in thin cytoplasmic layers of cells near the xylem vessels (xy-ve). D, Control cross section of a tumor, treated with 1% (w/v) rabbit serum and the secondary Alexa 488 antibody conjugate in the absence of the primary antibody against ABA, revealed no significant fluorescence signal. E to G, Images from bright-field microscopy. H and I, UV illuminated (488 nm) cross sections. E, Cross section of a tumor (tu) with a disrupted epidermis attached to an inflorescence stalk (stalk). The reddish color of the outer tumor cell layers marks Sudan-III-stained cells indicating suberin. Note that the stalk is covered by an intact epidermis, containing a cuticle (arrows). F, Closeup of outer cell layers from the tumor, attached to the host inflorescence stalk as shown in E with suberized cell walls (red) and of outer cell layers (G) from the host inflorescence stalk shown in E with a cuticle (red). H, Strong autofluorescence indicates aromatic compounds of lignified xylem vessels and outer cell layers of the tumor. I, Closeup of outer cell layers from the border between the tumor and inflorescence stalk shown in H. Autofluorescence marks cell walls of cells at the tumor surface but not at the surface of the stalk (H, white arrows). Bars: A, 5 mm; B, 100 μm; C, D, F, G, and I, 50 μm; E and H, 200 μm.
The Role of ABA in Agrobacterium-Mediated Crown Galls
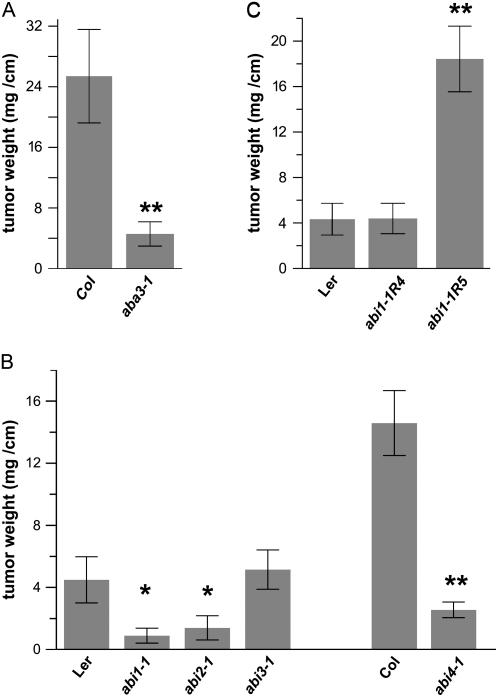
To investigate the role of ABA in Agrobacterium-Arabidopsis interactions we took advantage of Arabidopsis mutants impaired in ABA biosynthesis or signaling. Aba3-1, a mutant with strongly reduced ABA levels due to impairment in the final step of ABA biosynthesis, showed 6 times reduced tumor growth compared to appropriate wild-type controls, Columbia-0 (Col-0; Fig. 3A). Mutants affecting ABA-dependent signaling pathways, like the ABA-insensitive abi1-1, abi2-1, and abi4-1, exhibited reduced tumor growth (Fig. 3B). The ABA-insensitive mutant abi3-1, which affects the ABI3 transcription factor, had no effect on tumor growth. This indicated that ABA signaling, mediated by the ABA-dependent phosphatases ABI1 and ABI2, as well as the transcription factor ABI4, are relevant for tumor growth.
Figure 3.
Tumor growth on Arabidopsis mutants of ABA biosynthesis or signaling is impaired. A, Tumor growth on the ABA-deficient mutant aba3-1. on the ABA-insensitive mutants abi1, abi2, abi3, and abi4 (B; Mueller et al., 2006), and on the abi1 intragenic revertants abi1-1R4 and abi1-1R5 (C; Koornneef et al., 1984; Gosti et al., 1999). Tumor growth was determined per centimeter of infected inflorescence stalk 4 weeks after infection with Agrobacterium strain C58 and compared to the appropriate wild types. Error bars represent mean values (±sem) of seven to 20 plants per genotype; statistical analysis was performed using one-way ANOVA with Bonferroni post hoc test: P value < 0.05 (*); P value < 0.01 (**).
The ABA mutant phenotype could either be rescued genetically or by external application of ABA to deficient Arabidopsis plants to confirm the role of ABA in tumor development. However, as tumor growth requires 30 d and most of the externally supplied ABA would be metabolized and accumulate as phaseic and dihydrophaseic acid, which might produce unspecific side effects, revertants of abi1-1 were used (Gosti et al., 1999). The reduced tumor growth phenotype of abi1-1 mutant plants was rescued by the genetic revertants abi1-1R4 and abi1-1R5 (Fig. 3C). These revertants constitute double mutants, in which the original abi1-1 mutation is antagonized by additional mutations, resulting in attenuation of the abi1-1 mutant phenotype. Interestingly, the abi1-1R5 mutant, which displays a strong ABA-hypersensitive phenotype in the original drought stress screen of Gosti et al. (1999), also supported increased tumor growth in our assay (Fig. 3C).
Due to the complex phenotype of the genetic manipulations in these mutants we cannot distinguish between their potential effects on either efficiency of T-DNA transformation or the tumor growth following the successful T-DNA integration into the plant cell genome. To address this issue the efficiency of T-DNA integration of mutant and wild-type plants was compared by determining the transient expression of the reporter gene GUS driven by the 2× cauliflower mosaic virus 35S promoter. The staining of three representative Arabidopsis leaves (Supplemental Fig. S2A) or inflorescence stalk segments (Supplemental Fig. S2B) showed slight differences. These visual differences, however, were not significant when calculating the number of stained leaves (Supplemental Fig. S2C) or determining the activity of GUS, applying a fluorimetric assay (Supplemental Fig. S2D). In summary, the extensive transient expression experiments revealed no significant differences in transformation efficiency between wild-type and ABA mutant plants. Thus, these functional studies reveal a fundamental effect of ABA on tumor growth and not on efficiency of T-DNA integration.
Arabidopsis Tumors Accumulate Osmoprotectants and Express a Specific Pattern of ABA- and Drought-Responsive Genes
ABA signaling is well established as a mediator of drought stress adaptation in several plant models (Koornneef et al., 1984; Ooms et al., 1993; Verslues and Bray, 2006). The well-known altered response of abi1-1, abi2-1, and abi4-1 Arabidopsis mutants to drought stress might indicate a protective role of ABA against drought stress during crown gall development. In this respect it was found that crown galls accumulate high amounts of osmoprotectants. Pro was 12 times higher in tumors compared to inflorescence stalks (3.4 ± 0.07 versus 0.26 ± 0.08 μmol g−1 fresh weight [FW]). Additionally, α-aminoadipinic acid and Glu levels were 9 (1.3 ± 0.06 versus 0.14 ± 0.014 μmol g−1 FW) and 4 (4 ± 0.54 versus 1.3 ± 0.05 μmol g−1 FW) times higher, respectively (Deeken et al., 2006).
Analysis of gene expression in tumor tissue points to a distinct mechanism of drought acclimation in Agrobacterium-induced tumors, which differs from that of other well-investigated tissues, like leaves or roots. Several genes regulated by drought and/or ABA showed transcriptional activation in tumors: LEA (At2g46140), two ABA response-related genes (At5g23350, At5g08350), HVA22c, and the drought-inducible AtDI21 (Supplemental Table S1). The expression of AtDR4, a representative gene for those that are down-regulated by ABA and drought stress in Arabidopsis roots, was 6 times lower in the tumor than in reference tissues. Moreover, a strong induction of four out of 35 Arabidopsis aquaporin genes in tumor tissue was observed. Some of these water channel genes are known to be regulated by drought and/or ABA to protect cells from desiccation (Seki et al., 2001, 2002). In contrast, transcription of other well-known ABA- or drought-inducible marker genes, like RD20, COR47, several dehydrins, RAB18, ERD7, ALDEHYDE DEHYDROGENASE11, ALDH311, and LEA14 were significantly reduced in tumors (Supplemental Table S1; nomenclature according to The Arabidopsis Information Resource; www.arabidopsis.org/servlets).
Outer Cell Layers of Tumors Contain Suberin
Plants develop an epidermal cell layer that is covered by a cuticle to prevent substantial water loss. As the tumor is lacking an intact epidermis, modifications of the tumor surface that can inhibit desiccation were analyzed by use of specific staining and fluorescence techniques. The distribution of suberin in Arabidopsis tumors can be visualized by Sudan-III staining, a red dye commonly used to visualize aliphatic cell wall components. Cross sections of tumors revealed red staining in cell walls of the outer cell layers and a group of cells in the middle of the cross section representing the surface of an emarginated tumor area (Fig. 2, E and F). In addition, strong autofluorescence in response to blue light (488 nm, the typical spectrum of phenolic compounds) was also observed in these cell layers (Fig. 2, H and I). These results are in accordance with the chemical composition of suberin, which contains aliphatic as well as phenolic moieties, and suggest that cell walls of the tumors' outer surface incorporate suberin. In contrast, the cuticle of the inflorescence stalk revealed Sudan-III staining (Fig. 2, E [arrows] and G) but no blue light-induced autofluorescence (Fig. 2H, arrows), indicating the presence of aliphatic cutin. The autofluorescence of vascular tissue (Fig. 2H) that is not stained by Sudan-III (Fig. 2E) is most likely caused by lignin phenolics.
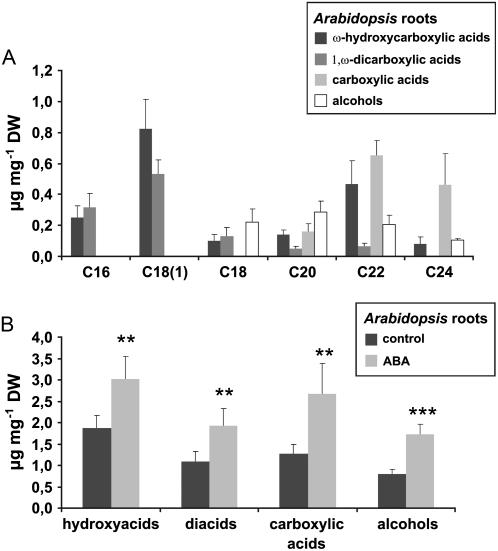
Chemical Composition of Tumor Suberin and Differential Expression of Genes Encoding Candidates for Suberin Biosynthesis
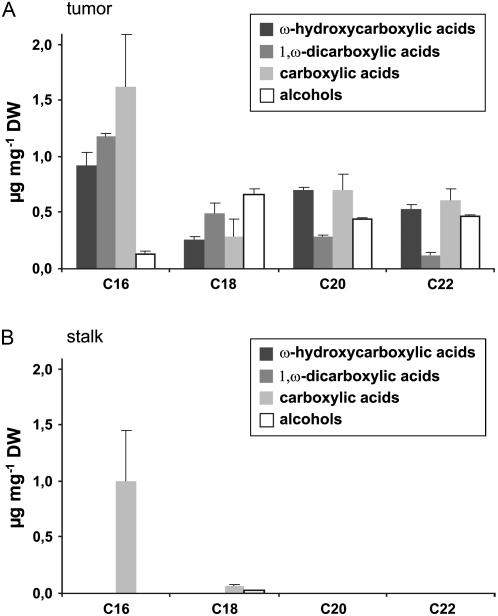
The aliphatic domain of suberin is essentially composed of monomeric units of long chain fatty acid derivatives (ω-hydrocarboxylic acids, 1,ω-dicarboxylic acids, carboxylic acids, and alcohols) that are connected through ester bonds (Kolattukudy, 1984). We found that deesterification of tumor tissue yields typical suberin monomers with the chain length extended from C16 to C22 (Fig. 4A). The total suberin content, as estimated from the sum of deesterified monomers, constituted 11.8 μg g−1 tumor dry weight. In contrast, deesterification of wounded inflorescence stalk tissue did not yield typical suberin monomers. The only fatty acid derivatives detected were stearic and palmitic acid, presumably originating from plasma membranes of cells from the stalk tissue (Fig. 4B).
Figure 4.
Identification of aliphatic suberin compounds in tumors. Substance classes and chain length distribution of aliphatic suberin constituents of tumor tissue (A) and inflorescence stalks (B) from nontumor plants. Results are given in micrograms monomer per milligram dry weight (DW). Bars represent mean values (±sd) of three independent experiments.
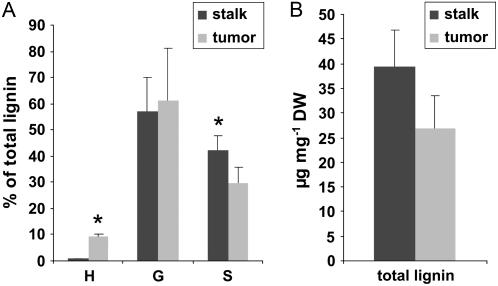
Like lignin, the aromatic moiety of suberin is composed of phenolic monomers with three distinct aromatic rings that differ in their number of methoxyl groups: p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S; Whetten and Sederoff, 1995; Lapierre et al., 1996; Schreiber et al., 1999). Suberized cell walls are often characterized by a higher H content in comparison to lignified tissue, and the yield of chemically degradable monomers is generally lower than in lignin (Zeier et al., 1999b). Using gas chromatography, we detected a substantial amount of H units in the tumor tissue (Fig. 5A). By comparison, isolates from inflorescence stalks that consisted to a major part of lignified vascular bundles were almost exclusively composed of G and S units and only contained traces of H. Moreover, the total content of degradable lignin monomers was lower in tumor than in the inflorescence stalk tissue (Fig. 5B). These findings indicate the presence of aromatic constituents of suberin in tumor tissue.
Figure 5.
Identification of aromatic suberin compounds in tumors. A, Ratios of H, G, and S to the total amount of lignin monomers released from inflorescence stalk (dark bars) or tumor tissue (light bars). B, Sum of lignin monomers given in micrograms lignin per milligram dry weight (DW). Bars represent mean values (±sd) of three independent experiments. Asterisks denote statistically significant differences (*: P < 0.05) between inflorescence stalk and tumor values (Student's t test).
In line with these results we found that the expression of genes encoding enzymes presumed to be involved in suberin biosynthesis was increased in tumors. The gene encoding the root-specific fatty acid ω-hydrolase CYP86A1 was 3 times higher expressed in tumors than in inflorescence stalk. In addition, the transcription of genes, encoding components of the biosynthesis of phenolic compounds such as PHE-AMMONIA LYASE1 and 4-COUMARATE-COA LIGASE2 as well as a peroxidase (At2g38390) and the LIPID TRANSFER PROTEIN2, putatively involved in lipid transport, were elevated in tumors (Supplemental Table S2; Kolattukudy, 1981; Bernards and Razem, 2001; Kunst et al., 2004; Duan and Schuler, 2005).
The histological and chemical analyses together with gene expression studies provide evidence that the outer cell layers of Agrobacterium-induced tumors are protected by suberized cell walls.
ABA Induces Suberization in Arabidopsis
To determine whether ABA is generally capable of inducing suberization in Arabidopsis, the impact of exogenous ABA on root suberization, a process readily feasible for experimental analysis, was investigated. Aliphatic suberin in Arabidopsis roots revealed structural similarity to tumor suberin (Fig. 6A). However, certain differences exist, as C18(1)-derived hydroxy- and diacids, which represent main components of many suberin polymers (Kolattukudy, 1984; Zeier and Schreiber, 1998; Zeier et al., 1999a; Franke et al., 2005) are present in root suberin but absent in tumor suberin. Additionally, the chain length distribution of root suberin is broader than that of tumor suberin (compare Figs. 4 and 6A). When roots of 2-week-old liquid cultures of Arabidopsis seedlings were treated with 10 μm ABA for 4 d, the root suberin content increased about 1.5- to 2-fold in comparison to water-treated control seedlings (Fig. 6B). This ABA-triggered increase in root suberization was similar for all substance classes (Fig. 6B) and no preferences for compounds of a certain chain length existed (data not shown). Thus, ABA proved to be capable of inducing suberin biosynthesis in Arabidopsis. These data strengthen our hypothesis that suberization of the outer cell layers of Arabidopsis tumors might be caused by high ABA levels inside the tumor.
Figure 6.
ABA-induced suberization of Arabidopsis roots. A, Substance classes and chain length distribution of aliphatic suberin constituents from Arabidopsis roots. B, Amounts of released aliphatic substance classes from control roots (dark bars) and roots treated with 10 μm of ABA (light bars). Results are given in micrograms per milligram of dry weight (DW). Bars represent mean values (±sd) of six independent experiments. Values for controls and ABA treatments are significantly different (**: P < 0.01, ***: P < 0.001, Student's t test) for all pairs of substance classes.
DISCUSSION
Agrobacterium-induced plant tumors show increased water loss, resulting in a redirection of water flow and nutrient transport necessary for tumor development (Schurr et al., 1996; Wächter et al., 2003). On the contrary, increased water loss causes tissue desiccation. Thus, successful tumor development requires a well-tuned balance between these opposing factors. Here we present evidence that the phytohormone ABA is able to regulate both of these processes. On the one hand, ABA has the potential to dampen water loss by inducing suberization of the tumor surface. On the other hand, ABA regulates stress genes to increase the osmotic potential in tumors and by that can direct water flow to the tumor. We propose a mechanism by which ABA maintains water potential in tumor tissue.
ABA Induces the Biosynthesis of a Protective Suberized Periderm-Like Layer
In Arabidopsis, like in other plant species, tumors lack an intact epidermis. As shown here, tumors seem to be protected against water loss by two to three suberized cell layers. Suberin of the tumor surface is structurally and chemically closely reminiscent of the periderm that constitutes the surface tissue of above-ground organs in the secondary growth stage of dicotyledonous plants and gymnosperms or of wound periderm (Schönherr and Ziegler, 1980; Schreiber et al., 2005). Besides a potential function of suberin in protection against pathogen infection, UV radiation, and nutrient depletion, suberized periderm tissue forms a considerable diffusion resistance against water vapor but is not necessarily a perfect impermeable barrier for water (Kolattukudy and Dean, 1974; Vogt et al., 1983; Nawrath, 2002; Franke et al., 2005; Schreiber et al., 2005). We therefore propose that suberization of the outer tumor cell layers is required for tumor development, since it is structurally capable of minimizing the loss of water.
The studies show that ABA induces suberin biosynthesis in Arabidopsis roots and that genes involved in this process were up-regulated in tumors. The root-specific NADPH-dependent P450-cytochrome monooxygenase CYP86A1, which catalyzes the biosynthesis of suberin aliphatic moieties, was 3 times elevated (Schuler and Werck-Reichhart, 2003; Duan and Schuler, 2005). The fact that its expression is up-regulated in tumors and its promoter contains several ABA- and drought-response elements (ABF/ABRE like) that have been shown to be ABA inducible (Benveniste et al., 1998), underlines our view that tumor suberization is indeed induced by ABA.
ABA Induces Drought Adaptations and Regulates Water Flow into Tumors
Although water loss is minimized in tumors, the tissue is still endangered by desiccation. Like in other species, tumors of Arabidopsis accumulate high levels of the stress hormone ABA (Mistrik et al., 2000; Veselov et al., 2003; Wächter et al., 2003). Accordingly, we identified ABA-dependent adaptive mechanisms on the level of gene regulation, as well as accumulation of osmoprotectants. A pattern of ABA-inducible genes that is different from the classical ABA-induced genes so far studied in seedlings, leaves, or roots was found to be up-regulated in tumors. This specific pattern includes a set of LEA and dehydrin genes. LEA proteins have been shown to be strongly accumulated in maturating seeds, but they can also be found in vegetative tissues. Their functional implication in drought protection has been worked out by Tolleter and colleagues (2007), prompting us to suggest this function for the tumor as well. During dehydration, these proteins can maintain the structure of endomembranes and other proteins by sequestration of ions, such as calcium. Additionally, these proteins have been shown to bind or replace water and act as molecular chaperones (Grelet et al., 2005; Mahajan and Tuteja, 2005; Tolleter et al., 2007). Thus, water stress in tumors is counteracted by an ABA-dependent up-regulation of stress genes.
Both Arabidopsis and Ricinus tumors accumulate high amounts of the osmoprotectant Pro. This amino acid is involved in elevation of the cell turgor and hence in maintaining the osmotic balance under drought stress conditions (Wächter et al., 2003). This role of Pro and other osmoprotectants in drought adaptation requires ABA signaling, since ABA-deficient mutants accumulate Pro to much lower levels when raised under drought or salt stress conditions (Xiong et al., 2001; Verslues and Bray, 2006). Analysis of tumor growth on mutant plants, exhibiting defects in ABA signaling, showed that phosphatases of the type 2C class affected in abi1 and abi2 plants, as well as the APETALA2 domain transcription factor affected in abi4 mutant plants, play a pivotal role during tumorigenesis (Leung et al., 1997; Finkelstein et al., 1998). The reduced tumor growth of these mutants, however, was not due to a reduction in T-DNA transformation efficiency. ABI1 and ABI2 are involved in adaptation of vegetative tissues to drought stress and have been shown to regulate Pro accumulation, together with ABI4, in response to a low water potential at reduced water availability (Verslues and Bray, 2006). In contrast, the transcription factor ABI3 has no effect on tumor development. As ABI3 has been shown to act downstream of the phosphatases ABI1 and ABI2, it may define a separate signaling pathway different from that controlled by ABI4 (Brady et al., 2003).
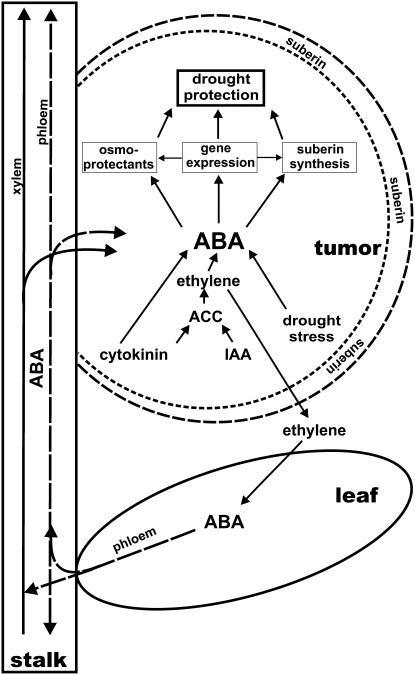
Taken together, we propose the following working model on how ABA might support tumor development (Fig. 7). While the physiological effects of ABA have already been discussed in previous paragraphs and are well in line with the effects discussed in the literature, our microarray analysis did not reveal any evidence for key enzymes of ABA metabolism being up-regulated in tumor tissue. Rather, immunolocalization of ABA suggests that this stress phytohormone might be translocated via the transpiration stream of the host plant into the tumor. This observation corresponds to earlier findings on the distribution of nitrate reductase activity, which was also found to be repressed in tumors; hence, amino acids have to be imported from the leaves as well (Deeken et al., 2006). From tumors of castor bean (Ricinus communis) it was proposed that the synthesis of auxin and cytokinin promotes an increased production of ethylene. Communication between the tumor and the host plant might well be provided by this gaseous factor, which in turn triggers synthesis of high ABA content in leaves of the host plant (Aloni et al., 1998; Wächter et al., 1999; Veselov et al., 2003). Since Arabidopsis tumors produce increased levels of the ethylene precursor ACC and develop very close to rosette leaves, ethylene diffusion seems very likely. High levels of ABA levels are found in Arabidopsis stalks above the rosette, as well as in tumor tissue close to vascular bundles. Thus, ethylene-induced ABA production in leaves might result in its transport via the phloem from leaves into tumors (Mistrik et al., 2000).
Figure 7.
Model of the role of ABA in tumor drought protection. In tumors T-DNA-encoded genes cause a higher cytokinin and indole acetic acid (IAA) content that induces ACC production followed by its conversion to ethylene. Emitted ethylene triggers ABA synthesis in tumors and host leaves. ABA is translocated from the rosette leaves into tumors via the phloem and xylem. In tumors ABA activates the expression of stress-responsive genes, synthesis of suberin, and causes elevated levels of osmoprotectants that result in suberization of outer cell layers and protect the tumor from desiccation.
This external supply of ABA has the potential to maintain the fine-tuned water balance in tumors autonomously. On the one hand, ABA-induced suberization of the tumor surface counteracts water loss. On the other hand, ABA-induced drought stress mechanisms, among them the accumulation of osmoprotectants, increase the osmotic potential and thus redirect water flow from the host plant to tumor. As both of these opposing effects are ABA triggered, communication between the tumor and plant via the gaseous factor ethylene can form a feed-forward loop to supply ABA from the plant to tumor.
MATERIALS AND METHODS
Plant Material and Tumor Induction
Plant cultivation and tumor induction were performed as previously described (Deeken et al., 2006). Wild-type Arabidopsis (Arabidopsis thaliana; ‘Wassilewskija’, ‘WS-2’; Col-0 Heynh.; Landsberg erecta) and mutant plants (aba3-1, abi1-1, abi2-1, abi3-1, abi4-1, abi1-1R4, and abi1-1R5) were inoculated with the nopaline-utilizing Agrobacterium tumefaciens strain C58noc (nopalin catabolism construction; no. 584; Max-Planck-Institute for Plant Breeding Research).
Arabidopsis Root Cultures and ABA Treatment
Surface-sterilized Col-0 seeds were transferred into an Erlenmeyer flask containing 100 mL of sterile Murashige and Skoog growth solution (Murashige and Skoog salts [Sigma] supplemented with Gamborg's B5 vitamins [Sigma] and 1% Suc) and shaken at 50 rpm. Seedlings that developed a sufficient amount of root material within 2 weeks were taken and ABA was added to a final concentration of 10 μm to induce suberization. Roots were harvested 4 d later. Control roots were treated with water.
Tissue Preparation and Immunolocalization of ABA
Four-week-old Arabidopsis tumors were infiltrated under vacuum and fixed for at least 24 h and further on treated as described in Schraut et al. (2004). For immunolocalization the green Alexa conjugate 488 (488 goat anti-mouse IgG, H+L, Molecular Probes; excitation 488 nm, emission 506–512 nm) was used as a secondary antibody as described previously (Langhans et al., 2001; Veselov et al., 2003; Schraut et al., 2004). Control cross sections, demonstrating the specificity of ABA-derived signals, were performed in the same way as shown in Schraut et al. (2004). Sections were stained with toluidine blue to quench the autofluorescence of the lignified cell walls and inspected with a confocal laser-scanning microscope (LEICA-TCS-SP).
Suberin Localization
Tumor cross sections as used for ABA immunolocalization were stained with a saturated solution of Sudan-III in 92% ethanol (w/v) for 10 min at 70°C, then washed with glycerol:water (1:1, v/v). Sections were examined using a Leica DMR microscope with bright-field illumination or fluorescence excitation of 488 nm using a two-dimensional blue filter (Leica).
Chemical Analysis of Suberin and Lignin
Analytical determination of suberin and lignin in isolated plant tissue (1–2 mg dry weight) was performed according to Zeier et al. (1999b). For identification and quantification of the reaction products gas chromatography was applied as described in detail by Zeier and Schreiber (1997).
Determination of ABA Content
Free ABA and ABA released from its conjugates was analyzed by ELISA as described earlier (Jiang et al., 2004). Recoveries of ABA during the purification procedures were checked routinely using radioactive ABA and found to be more than 95%. The immunochemicals were generously supplied by Professor Weiler, Ruhr Universität Bochum (Germany).
Determination of ACC Content
Plant material (200 mg, stored at −80°C) was mixed with 990 μL of methanol and 10 μL of acetic acid, homogenized, and extracted using a vibrating ball mill MM 301 (Retsch) for 3 min. Samples were centrifuged and the supernatant was dried in a vacuum centrifuge. As internal standard 50 ng norvaline was added. Samples were derivatized with pentafluorobenzyl (PFB) bromide and purified by vapor phase extraction prior to gas chromatography-mass spectrometric analysis in the negative ion chemical ionization mode as described recently (Mueller et al., 2006). Molecular anions, [M-PFB]−, of the double PFB ester, amide derivatives of ACC, and norvaline were monitored in the single ion monitoring mode and used for quantification.
Agrobacterium-Mediated Transient Transformation
For transient transformation of Arabidopsis, Agrobacterium GV3101 (pMP90; Koncz and Schell, 1986) was used. This strain harbored the binary plasmid pMDC164 for expression of GUS under control of the 2× cauliflower mosaic virus 35S promoter and was mixed with strain 19 K (Latz et al., 2007) before infiltration to prevent gene silencing. Growth of agrobacteria and infiltration (agroinfiltration) into leaves of 2- to 3-month-old Arabidopsis leaves or injection into the base of inflorescence stalks of 3- to 4-month-old plants was carried out as described in Zipfel et al. (2006) with minor modifications. Qualitative and quantitative measurements of GUS activity 4 to 7 d after infiltration with agrobacteria were performed according to Jefferson et al. (1987). For more detailed protocol see Supplemental Materials and Methods S1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression pattern of ABA biosynthesis genes in tumors
Supplemental Figure S2. Transient GUS expression in adult Arabidopsis leaves or inflorescence stalks of mutants, impaired either in ABA synthesis or signaling.
Supplemental Table S1. Differential expression of ABA- and drought-regulated genes in Arabidopsis tumors.
Supplemental Table S2. Differential expression of genes predicted to encode enzymes of suberin biosynthesis in Arabidopsis tumors.
Supplemental Materials and Methods S1. Detailed protocols.
Supplementary Material
Acknowledgments
We are grateful to B. Roeger and T. Latz for excellent technical support and Professor C.I. Ullrich-Eberius (Institut für Botanik, Technische Hochschule Darmstadt, Germany) for supplying us with the A. tumefaciens strain.
This work was supported by the Deutsch Forschungsgemeinschaft (grant no. SFB567, project B5, to R.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Rosalia Deeken (deeken@botanik.uni-wuerzburg.de).
The online version of this article contains Web-only data.
References
- Aloni R, Pradel KS, Ullrich CI (1995) The 3-dimensional structure of vascular tissues in Agrobacterium tumefaciens-induced crown galls and in the host stems of Ricinus-communis L. Planta 196 597–605 [Google Scholar]
- Aloni R, Wolf A, Feigenbaum P, Avni A, Klee HJ (1998) The never ripe mutant provides evidence that tumor-induced ethylene controls the morphogenesis of Agrobacterium tumefaciens-induced crown galls on tomato stems. Plant Physiol 117 841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I, Tijet N, Adas F, Philipps G, Salaün JP, Durst F (1998) CYP86A1 from Arabidopsis thaliana encodes a cytochrome P450-dependent fatty acid omega-hydroxylase. Biochem Biophys Res Commun 243 688–693 [DOI] [PubMed] [Google Scholar]
- Bernards MA, Razem FA (2001) The poly(phenolic) domain of potato suberin: a non-lignin cell wall bio-polymer. Phytochemistry 57 1115–1122 [DOI] [PubMed] [Google Scholar]
- Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, et al (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Sarkar SF, Bonetta D, McCourt P (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34 67–75 [DOI] [PubMed] [Google Scholar]
- Cottle W, Kolattukudy PE (1982) Abscisic acid stimulation of suberization: induction of enzymes and deposition of polymeric components and associated waxes in tissue cultures of potato tuber. Plant Physiol 70 775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeken R, Engelmann JC, Efetova M, Czirjak T, Müller T, Kaiser WM, Tietz O, Krischke M, Mueller MJ, Palme K, et al (2006) An integrated view of gene expression and solute profiles of Arabidopsis tumors: a genome-wide approach. Plant Cell 18 3617–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Schuler MA (2005) Differential expression and evolution of the Arabidopsis CYP86A subfamily. Plant Physiol 137 1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2005) Apoplastic polyesters in Arabidopsis surface tissues—a typical suberin and a particular cutin. Phytochemistry 66 2643–2658 [DOI] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelet J, Benamar A, Teyssier E, Avelange-Macherel M-H, Grunwald D, Macherel D (2005) Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol 137 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Jeschke WD, Hartung W (2004) Abscisic acid (ABA) flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite abscisic acid relations. J Exp Bot 55 2323–2329 [DOI] [PubMed] [Google Scholar]
- Kado CI (1984) Phytohormone-mediated tumorigenesis by plant pathogenic bacteria. In DPS Verma, T Hohn, eds, Genes Involved in Microbe-Plant Interactions. Springer Verlag, Heidelberg, Germany, pp 311–336
- Kolattukudy P, Dean B (1974) Structure, gas chromatographic measurement, and function of suberin synthesized by potato tuber tissue slices. Plant Physiol 54 116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE (1981) Structure, biosynthesis and biodegradation of cutin and suberin. Annu Rev Plant Physiol 32 539–567 [Google Scholar]
- Kolattukudy PE (1984) Biochemistry and function of cutin and suberin. Can J Bot 62 2918–2933 [Google Scholar]
- Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204 383–396 [Google Scholar]
- Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic-acid insensitive mutants of Arabidopsis thaliana. Physiol Plant 61 377–383 [Google Scholar]
- Kunst L, Samuels AL, Jetter R (2004) The plant cuticle: formation and structure of epidermal surfaces. In D Murphy, ed, Plant Lipids—Biology, Utilisation and Manipulation. Blackwell Scientific, Oxford, pp 270–302
- Langhans M, Ratajczak R, Lützelschwab M, Michalke W, Wächter R, Fischer-Schliebs E, Ullrich CI (2001) Immunolocalization of plasma-membrane H+-ATPase and tonoplast-type pyrophosphatase in the plasma membrane of the sieve element-companion cell complex in the stem of Ricinus communis L. Planta 213 11–19 [DOI] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Negrel J (1996) The phenolic domain of potato suberin: structural comparison with lignins. Phytochemistry 42 949–953 [Google Scholar]
- Latz A, Ivashikina N, Fischer S, Ache P, Sano T, Becker D, Deeken R, Hedrich R (2007) In planta AKT2 subunits constitute a pH- and Ca2+-sensitive inward rectifying K+ channel. Planta 225 1179–1191 [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444 139–158 [DOI] [PubMed] [Google Scholar]
- Mistrik I, Pavlovkin J, Wächter R, Pradel KS, Schwalm K, Hartung W, Mathesius U, Stöhr C, Ullrich CI (2000) Impact of Agrobacterium tumefaciens-induced stem tumors on NO3− uptake in Ricinus communis. Plant Soil 226 87–98 [Google Scholar]
- Mueller MJ, Mene-Saffrane L, Grun C, Karg K, Farmer EE (2006) Oxylipin analysis methods. Plant J 45 472–489 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56 165–185 [DOI] [PubMed] [Google Scholar]
- Nawrath C (2002) The biopolymers cutin and suberin. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD [DOI] [PMC free article] [PubMed]
- Ooms J, Leon-Kloosterziel KM, Bartels D, Koornneef M, Karssen CM (1993) Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana (a comparative study using abscisic acid-insensitive abi3 mutants). Plant Physiol 102 1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr J, Ziegler H (1980) Water permeability of Betula periderm. Planta 147 345–354 [DOI] [PubMed] [Google Scholar]
- Schraut D, Ullrich CI, Hartung W (2004) Lateral ABA transport in maize roots (Zea mays): visualization by immunolocalization. J Exp Bot 55 1635–1641 [DOI] [PubMed] [Google Scholar]
- Schreiber L, Franke R, Hartmann K (2005) Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 220 520–530 [DOI] [PubMed] [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot 50 1267–1280 [Google Scholar]
- Schuler MA, Werck-Reichhart D (2003) Functional genomics of P450s. Annu Rev Plant Biol 54 629–667 [DOI] [PubMed] [Google Scholar]
- Schurr U, Schuberth B, Aloni R, Pradel KS, Schmundt D, Jahne B, Ullrich CI (1996) Structural and functional evidence for xylem-mediated water transport and high transpiration in Agrobacterium tumefaciens-induced tumors of Ricinus communis. Bot Acta 109 405–411 [Google Scholar]
- Seki M, Ishida J, Narusaka M, Fujita M, Nanjo T, Umezawa T, Kamiya A, Nakajima M, Enju A, Sakurai T, et al (2002) Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct Integr Genomics 2 282–291 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday CL, Dean BB, Kolattukudy PE (1978) Suberization: inhibition by washing and stimulation by abscisic acid in potato disks and tissue culture. Plant Physiol 61 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF, Nutter R, Montoya AL, Gordon MP, Nester EW (1980) Integration and organization of Ti plasmid sequences in crown gall tumors. Cell 19 729–739 [DOI] [PubMed] [Google Scholar]
- Tolleter D, Jaquinod M, Mangavel C, Passirani C, Saulnier P, Manon S, Teyssier E, Payet N, Avelange-Macherel M-H, Macherel D (2007) Structure and function of a mitochondrial late embryogenesis abundant protein are revealed by desiccation. Plant Cell 19 1580–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich CI, Aloni R (2000) Vascularization is a general requirement for growth of plant and animal tumours. J Exp Bot 51 1951–1960 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Bray EA (2006) Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J Exp Bot 57 201–212 [DOI] [PubMed] [Google Scholar]
- Veselov D, Langhans M, Hartung W, Aloni R, Feussner I, Götz C, Veselova S, Schlomski S, Dickler C, Bachmann K, et al (2003) Development of Agrobacterium tumefaciens C58-induced plant tumors and impact on host shoots are controlled by a cascade of jasmonic acid, auxin, cytokinin, ethylene and abscisic acid. Planta 216 512–522 [DOI] [PubMed] [Google Scholar]
- Vogt E, Schönherr J, Schmidt HW (1983) Water permeability of periderm membranes isolated enzymatically from potato tubers (Solanum tuberosum L.). Planta 158 294–301 [DOI] [PubMed] [Google Scholar]
- Wächter R, Fischer K, Gäbler R, Kühnemann F, Urban W, Bögemann GM, Voesenek L, Blom C, Ullrich CI (1999) Ethylene production and ACC-accumulation in Agrobacterium tumefaciens-induced plant tumours and their impact on tumour and host stem structure and function. Plant Cell Environ 22 1263–1273 [Google Scholar]
- Wächter R, Langhans M, Aloni R, Götz S, Weilmünster A, Koops A, Temguia L, Mistrik I, Pavlovkin J, Rascher U, et al (2003) Vascularization, high-volume solution flow, and localized roles for enzymes of sucrose metabolism during tumorigenesis by Agrobacterium tumefaciens. Plant Physiol 133 1024–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten R, Sederoff R (1995) Lignin biosynthesis. Plant Cell 7 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13 2063–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217 1214–1222 [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39 439–473 [Google Scholar]
- Zeier J, Goll A, Schreiber L, Yokoyama M, Karahara I (1999. a) Structure and chemical composition of endodermal and rhizodermal/hypodermal walls of several species. Plant Cell Environ 22 271–279 [Google Scholar]
- Zeier J, Schreiber L (1997) Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata: identification of the biopolymers lignin and suberin. Plant Physiol 113 1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Schreiber L (1998) Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledoneous species: chemical composition in relation to fine structure. Planta 206 349–361 [Google Scholar]
- Zeier J, Schreiber L, Ruel K, Ryser U (1999. b) Chemical analysis and immunolocalisation of lignin and suberin in endodermal and hypodermal/rhizodermal cell walls of developing maize (Zea mays L.) primary roots. Planta 209 1–12 [DOI] [PubMed] [Google Scholar]
- Zhu C, Schraut D, Hartung W, Schäffner AR (2005) Differential responses of maize MIP genes to salt stress and ABA. J Exp Bot 56 2971–2981 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125 749–760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.