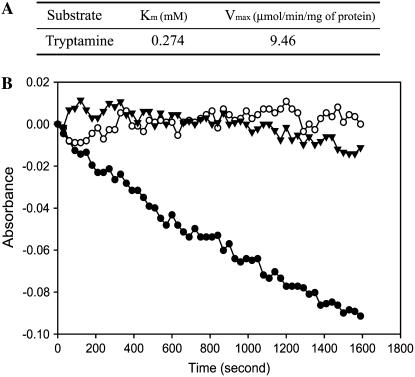
Figure 7.
Characteristics of typtamine-dependent oxidation of NADPH catalyzed by MBP:YUCCA6 expressed in E. coli. A, Kinetic parameters (Km and Vmax) for typtamine-dependent oxidation of NADPH catalyzed by YUCCA6-maltose-binding fusion proteins. The kinetic parameters were calculated from initial velocity measurements by following substrate-dependent consumption of NADPH at pH 8.0 with 0.1 mm, 1.0 mm, 2 mm, 2.5 mm, 5 mm, and 10 mm concentrations of tryptamine described in “Materials and Methods.” Reactions were carried out for 4 min, and initial velocities were estimated from slopes of consumption curves. B, Velocity of NADPH consumption catalyzed by MBP (control) and YUCCA6-maltose-binding fusion proteins using 2 mm tryptamine and MBP:YUCCA6 using 1 mm IAN. MBP (white circle) and MBP:YUCCA6 (black circle) proteins were incubated in reaction mixtures containing 0.1 mm NADPH and 2 mm tryptamine in 50 mm potassium phosphate buffer, pH 8.0, at 22°C for 30 min. Velocity of MBP:YUCCA6 proteins at 1 mm IAN (black triangle) was measured in the same conditions using IAN instead of tryptamine.