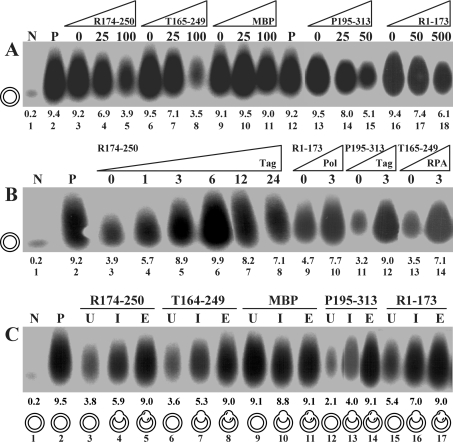
Figure 2. Influence of peptides on the monopolymerase system.
(A) Titration of peptides. Increasing amounts of indicated peptides displayed as fold molar excess over 6.25 pmol of Tag were titrated into the reaction at the onset of monopolymerase reactions. (B) Back-titration of replication factors. Replication factors were added back at the indicated amounts at the onset of the reactions. Molar excess of peptides over Tag was 100-fold (for R1-173, 500-fold). (C) Kinetics of inhibition. Indicated peptides were added to the reaction mixture at a 100-fold (500-fold for R1-173) molar excess over Tag before unwinding (U), primer synthesis (I) and primer elongation (E). The different template configurations present at the time at which the peptides were administered were under-wound (U), primed (I) and primer-extended (E) plasmid DNAs, which are symbolized for each reaction. Lanes N and P represent negative and positive controls in the absence and presence of Tag. The numbers above lane numbers denote the amount of nucleotides incorporated (in pmol).