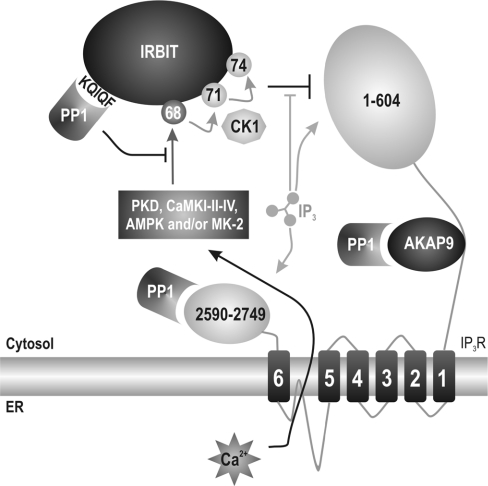
Figure 7. Proposed functions of PP1 in IP3R signalling.
Proposed model in which IP3 competes with IRBIT for binding to the N-terminus of IP3R (residues 1–604). Binding of IP3 is favoured as it has a higher affinity for the IP3R. The binding of IP3 induces Ca2+ release from the ER (endoplasmic reticulum). Ca2+ can then activate the phosphorylation cascade on Ser68, Ser71 and Ser74. We propose that PKD, CaMKI/II/IV, AMPK and/or MK-2 are probable candidates to phosphorylate Ser68 in response to Ca2+ release. PP1 is bound to the N-terminal KQIQF motif on IRBIT, and this binding of PP1 allows for efficient dephosphorylation of Ser68. PP1 also directly interacts with the C-terminus of the IP3R (residues 2590–2749) and indirectly via AKAP9 to the regulatory domain of the IP3R.