Abstract
Obesity and diabetes are associated with increased fatty acid availability in excess of muscle fatty acid oxidation capacity. This mismatch is implicated in the pathogenesis of cardiac contractile dysfunction and also in the development of skeletal-muscle insulin resistance. We tested the hypothesis that ‘Western’ and high fat diets differentially cause maladaptation of cardiac- and skeletal-muscle fatty acid oxidation, resulting in cardiac contractile dysfunction. Wistar rats were fed on low fat, ‘Western’ or high fat (10, 45 or 60% calories from fat respectively) diet for acute (1 day to 1 week), short (4–8 weeks), intermediate (16–24 weeks) or long (32–48 weeks) term. Oleate oxidation in heart muscle ex vivo increased with high fat diet at all time points investigated. In contrast, cardiac oleate oxidation increased with Western diet in the acute, short and intermediate term, but not in the long term. Consistent with fatty acid oxidation maladaptation, cardiac power decreased with long-term Western diet only. In contrast, soleus muscle oleate oxidation (ex vivo) increased only in the acute and short term with either Western or high fat feeding. Fatty acid-responsive genes, including PDHK4 (pyruvate dehydrogenase kinase 4) and CTE1 (cytosolic thioesterase 1), increased in heart and soleus muscle to a greater extent with feeding a high fat diet compared with a Western diet. In conclusion, we implicate inadequate induction of a cassette of fatty acid-responsive genes, and impaired activation of fatty acid oxidation, in the development of cardiac dysfunction with Western diet.
Keywords: cardiac function, cardiac metabolism, diet-induced obesity, futile cycling, gene expression, triacylglycerol
Abbreviations: CPT, carnitine palmitoyltransferase; CTE1, cytosolic thioesterase 1; GLUT4, glucose transporter 4; MTE1, mitochondrial thioesterase 1; MV˙O2, myocardial oxygen consumption; NEFA, non-esterified fatty acid; PDHK, pyruvate dehydrogenase kinase; PPARα, peroxisome-proliferator-activated receptor α; ROS, reactive oxygen species; UCP3, uncoupling protein 3
INTRODUCTION
Metabolic adaptation to changes in the physiological environment is a requisite for normal muscle function. The contractile dysfunction of the heart in obesity and diabetes is inextricably linked to dysfunction of cardiac metabolism [1]. With the growing prevalence of obesity [2] and reports of cardiac dysfunction with obesity in humans and animal models [3–5], it should be of interest to characterize metabolic derangement within the cardiomyocyte in response to dietary lipid challenge, which may be responsible for cardiac dysfunction. Adaptations in lipid metabolism in response to specific lipid components of the diet (e.g. the composition of saturated and unsaturated fatty acids) are important in the maintenance of normal cardiac function [6,7]. However, the importance of the source of excess calories in the diet is in need of further investigation.
The main mechanism by which heart and skeletal muscle adapt to a high fat environment is ligand activation of the PPARα (peroxisome-proliferator-activated receptor α) transcription factor [8]. PPARα activation leads to transcriptional activation of multiple enzymes in the pathways of fatty acid utilization. PPARα activation increases fatty acid oxidation and protects the heart from substrate-induced contractile dysfunction when there is an oversupply of fatty acids and triacylglycerols. Conversely, evidence also exists suggesting that inappropriate activation of distinct end-processes of PPARα activation in the heart (e.g. fatty acid storage) can be detrimental in the face of specific fatty acid challenges, such as consumption of a diet rich in saturated long-chain fatty acids [9]. Previously published studies strongly suggest the necessity of tight regulation of multiple PPARα-regulated processes in the high fat environment, such as mitochondrial uncoupling [10–13]. The differential activation of PPARα-mediated processes in the heart in the presence of long-term exposure to a high fat environment is not entirely known. It remains unclear which of the processes of fatty acid utilization (e.g. intramyocardial storage compared with fatty acid-mediated uncoupling of oxidative phosphorylation) are protective and which of them are detrimental to cardiac function.
Understanding the regulation of glucose utilization in the heart and skeletal muscle of obese or diabetic individuals is linked to understanding fat utilization. In the normal heart, a high fat environment promotes fatty acid oxidation, which, in turn, inhibits glucose oxidation [14]. This condition may be detrimental in the face of acute or prolonged stress. Substantial evidence suggests that glucose utilization is critical to maintain contractile function when the heart is stressed [15,16]. At the same time, the importance of dietary carbohydrates in the development of glucotoxicity in tissues needs to be considered, particularly when it is related to states of high fat supply such as diabetes and obesity.
Skeletal muscle also adapts and maladapts to a high calorie environment. A major role of skeletal muscle is the disposal of glucose. Previous work in a rat model of diet-induced obesity demonstrates that skeletal muscle becomes insulin-resistant with long term (up to 30 weeks) high fat feeding, while insulin signalling was preserved in the short term (8 weeks) [17]. Recent work in C57BL/6 mice has demonstrated that high fat feeding causes cardiac muscle to develop insulin resistance before skeletal-muscle insulin resistance, and both these maladaptive events antedate the development of cardiac dysfunction [18].
The present study focused on a diet-induced obesity model in the Wistar rat. While there are multiple genetic models of cardiac lipotoxicity in rats (e.g. Zucker diabetic fatty rat [5] and Zucker obese rat [19]) and mice (e.g. ob/ob [20] and db/db mice [21]), we decided to characterize the adaptiveness of substrate oxidation and PPARα activation in Wistar rats fed on a ‘Western’ or high fat diet (45 or 60% calories from fat respectively) for up to 48 weeks. We reasoned this approach is most akin to the primary cause of obesity observed in the Western world today. We have addressed three main objectives: (i) to characterize cardiac function and cardiac metabolic flexibility in response to both a chronic high fat environment and with an acute stress [adrenaline (epinephrine) stimulation in the isolated working heart]; (ii) to determine the transcriptional regulation of key PPARα-regulated genes in response to either ‘Western’ or high fat diets; and (iii) to determine the relationship between cardiac- and skeletal-muscle metabolic adaptation, both at the transcriptional and substrate oxidation levels, to Western or high fat diets over the course of 48 weeks. Here, we report a decrease in cardiac contractile function with Western diet (but not with high fat diet), which occurs in a state of submaximal induction of both fatty acid oxidation and a cassette of fatty acid-responsive genes.
EXPERIMENTAL
Rats and feeding
All procedures were approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston. Male Wistar rats (6 weeks old, 150 g) were obtained from Harlan (Indianapolis, IN, U.S.A.) and were housed at the Animal Care Center of the University of Texas Medical School at Houston under controlled conditions. Briefly, rats were housed in pairs at 23±1 °C with a 12 h light/12 h dark cycle. The rats were allowed to acclimatize for 2 weeks prior to initiation of ‘low fat’ [10% calories from fat, Research Diets no. D12450B (Research Diets, New Brunswick, NJ, U.S.A.)], ‘Western’ (45% calories from fat, Research Diets no. D12451) or ‘high fat’ (60% calories from fat, Research Diets no. D12492) diet feeding ad libitum. The calorie composition of the diets is given in Table 1. The low fat diet is similar in macronutrient composition to the standard laboratory chow (e.g. LabDiet® Rodent Diet 5001 contains as a percentage of total calories: 28% protein, 60% carbohydrate and 12% fat). Each rat was weighed weekly.
Table 1. Calorie composition of the diets used in the present study.
| Calorie composition (%) | |||
|---|---|---|---|
| Low fat diet | Western diet | High fat diet | |
| Protein | 20 | 20 | 20 |
| Carbohydrate | 70 | 35 | 20 |
| Fat | 10 | 45 | 60 |
| Saturated fat | 2.5 | 16 | 22 |
| Monounsaturated fat | 3.5 | 20 | 28 |
| Polyunsaturated fat | 4.0 | 8.6 | 10 |
In the first set of studies, rats (n=250) were killed (6±1.5 h into the dark phase) and the heart was perfused ex vivo after 1 day and 1 week (acute term), 4 and 8 weeks (short term), 16 and 24 weeks (intermediate term) and 32 and 48 weeks (long term) on the feeding protocols. At the time of killing, plasma was obtained and soleus muscle was harvested; both were snap-frozen and stored at −80 °C until further analysis. Tibia length was also measured.
In the second set of studies, rats (n=200), subjected to the same feeding protocol as above, were killed (6±1.5 h into the dark phase) and both soleus muscles were isolated for determination of insulin-mediated substrate metabolism ex vivo. At the same time, both ventricles of the heart were isolated, weighed, snap-frozen and stored at −80 °C until further analysis.
Determination of cardiac power and cardiac substrate oxidation
In order to determine the metabolic adaptation and cardiac function following high fat feeding, hearts were perfused using the isolated working heart preparation as described previously [22,23]. Briefly, rats were anaesthetized with chloral hydrate (300 mg/kg body weight intraperitoneal). Hearts were removed rapidly, placed in ice-cold Krebs–Henseleit buffer [24] (118.5 mM NaCl, 4.75 mM KCl, 1.18 mM KH2PO4, 1.18 mM MgSO4, 2.54 mM CaCl2 and 25 mM NaHCO3) and initially perfused in the Langendorff mode with Krebs–Henseleit buffer equilibrated with 95:5 O2/CO2. This was followed by a 40-min non-recirculating perfusion at 37 °C in the working mode (15 cm water filling pressure and 100 cm water afterload) with Krebs–Henseleit buffer equilibrated with 95:5 O2/CO2 containing 5 mM D-glucose {plus 20 μCi/l [U-14C]glucose (MP-Biomedicals, Solon, OH, U.S.A.)}, 0.4 mM sodium oleate {plus 30 μCi/l [9,10-3H]oleate (Sigma–Aldrich, St. Louis, MO, U.S.A.)} bound to 1% BSA (fraction V, fatty acid-free; Serologicals, Norcross, GA, U.S.A.; dialysed), and 40 μU (microunits)/ml insulin (Sigma–Aldrich). After 20 min, 1 μM adrenaline (Sigma–Aldrich) was added and the afterload increased to 140 cm water. The perfusion was allowed to continue for an additional 20 min, after which the ventricles were dissected and freeze-clamped with aluminium tongs cooled in liquid nitrogen. Cardiac power, myocardial oxygen consumption (MV˙O2), cardiac efficiency, glucose oxidation flux and oleate oxidation flux were determined as described previously [23].
Determination of soleus muscle insulin-mediated substrate utilization
In order to determine the metabolic adaptation of soleus muscle to Western or high fat feeding, isolated soleus muscles were incubated as described previously [25]. Briefly, rats were anaesthetized with chloral hydrate (300 mg/kg body weight intraperitoneal) and four soleus muscle strips (18–40 mg) were isolated from each rat, tied to stainless steel clips and incubated at 37 °C in a shaking water bath in a 25-ml Erlenmeyer flask with 3 ml of Krebs–Henseleit buffer containing 5 mM D-glucose, 0.4 mM sodium oleate bound to 1% BSA (dialysed), and 1 μU/ml insulin, which was continuously equilibrated with 95:5 O2/CO2. After 45 min, soleus muscle strips were transferred to 3 ml of Krebs–Henseleit buffer containing 5 mM D-glucose (plus 500 μCi/l [U-14C]glucose), 0.4 mM sodium oleate (plus 750 μCi/l [9,10-3H]oleate) bound to 1% BSA (dialysed), and either 100 μU/ml (submaximal) or 1000 μU/ml (supra-maximal) insulin, which was equilibrated with 95:5 O2/CO2 for 30 min and then sealed for the remaining 30 min. After incubation in a shaking water bath (37 °C), muscle strips were removed, snap-frozen and stored at −80 °C for further analysis. The flux of glucose oxidation, oleate oxidation, radioactive lactate release and radioactive glycogen production were determined as previously described [25].
Determination of metabolic gene transcript number in heart and soleus muscle
In order to determine the transcript levels of key metabolic genes involved in adaptation to high fat feeding in heart and soleus muscle, quantitative RT (reverse transcriptase)–PCR was performed as described previously [26]. Primer and probe sequences have been published previously [12,13,27,28]. Standard RNA was made for all assays by the T7 polymerase method (Ambion, Austin, TX, U.S.A.), using total RNA isolated from rat hearts. The correlation between the Ct (the number of PCR cycles required for the fluorescent signal to reach a detection threshold) and the amount of standard RNA was linear over a 5-log range for all assays (results not shown). Absolute level of expression is represented as transcripts per nanogram of total RNA.
Determination of plasma metabolite levels
Blood (2 ml) was taken from the inferior vena cava prior to harvesting the heart, transferred to a tube containing EDTA and spun in a centrifuge at 380 g for 20 min. Then, plasma was transferred to an Eppendorf tube and stored at −80 °C until further analysis. NEFA (non-esterified fatty acid) concentration was determined using a commercially available kit (NEFA C; Wako Chemicals, Richmond, VA, U.S.A.). Specimen blanks were prepared for all samples to correct for possible haemolysis. Blood glucose concentration was determined using a commercial monitor (FreeStyle; Abbott Laboratories, Abbott Park, IL, U.S.A.).
Determination of cardiac triacylglycerol content
To determine the accumulation of triacylglycerol within the myocardium in response to distinct diets, cardiac triacylglycerols were extracted from approx. 50 mg of heart tissue in chloroform/methanol [29] and quantified using a commercially available kit (Sigma–Aldrich).
Determination of cardiac protein carbonyl content
To determine the carbonyl content of myocardial proteins as a marker of oxidative damage, proteins were derivatized with 2,4-dinitrophenylhydrazine and measured with a spectrophotometer [30,31]. Briefly, 0.7 ml of 10 mM 2,4-dinitrophenylhydrazine in 2 M HCl was added to 1 mg of protein in 0.2 ml of protein extraction buffer [30 mM Hepes, 2.5 mM EGTA, 2.5 mM EDTA, 20 mM KCl, 40 mM 2-glycerophosphate, 40 mM NaF, 4 mM NaPPi (sodium pyrophosphate), 0.1% Nonidet P40 and 10% glycerol]. Derivative formation was performed for 20 min at room temperature (23 °C) with constant shaking. Proteins were precipitated with the addition of an equal volume of 20% (v/v) trichloroacetic acid and centrifugation at 16060 g for 5 min in a desktop centrifuge. The pellet was then washed with three washes of 1 ml of ethyl acetate/ethanol (1:1). The pellet was resuspended in 1 ml of 6 M guanidinium chloride and absorbance was read at 280 and 370 nm. Carbonyl content (nanomoles) was determined by the A370×45.5, which was corrected for background absorbance of the preparation [32]. Protein content was determined by comparison with the absorbance at 280 nm of a standard curve of known concentrations of BSA in 6 M guanidine.
Statistical analysis
Heart perfusion data are presented as means±S.E.M. at time 20 min (baseline data) or time 40 min (acute adrenaline stimulation data) of the perfusion and were analysed with SPSS version 13.0 (SPSS Inc., Chicago, IL, U.S.A.). Perfusion data were analysed using repeated measure ANOVA between two groups. All other results are presented as means±S.E.M. and statistically significant differences were calculated by the Student's t test. For all analyses, P<0.05 was considered statistically significant.
RESULTS
Rats fed on Western and high fat diets gain more weight than rats fed on a low fat diet
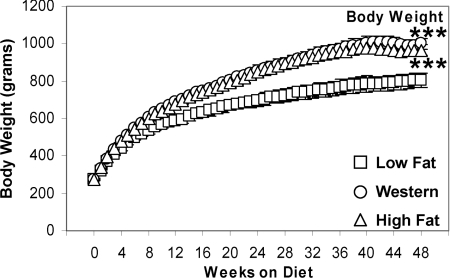
Rats fed on Western or high fat diet gained more weight (+33%) compared with rats fed on a low fat diet (P<0.001; Figure 1). There was no difference in tibia lengths (results not shown) between the groups, suggesting it is unlikely that alterations in lean body mass account for changes in total body weight. Ventricular weights did not differ between groups at all time points investigated (results not shown).
Figure 1. Body weight is increased with Western and high fat diet feeding.
Values are means±S.E.M. for 14–22 independent experiments per group. Open squares (□) represent low fat diet-fed rats, open circles (○) represent Western diet-fed rats and open triangles (Δ) represent high fat diet-fed rats. ***, P<0.001 compared with low fat diet for the entire feeding protocol.
Western or high fat feeding results in increased plasma NEFA with no net accumulation of cardiac triacylglycerols or protein carbonylation content
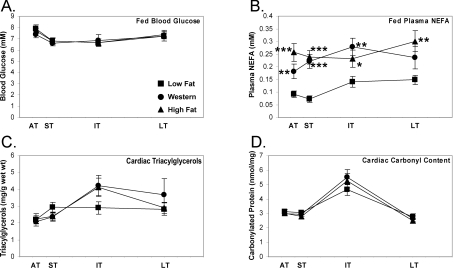
There was no significant difference in blood glucose concentrations of fed rats between all groups at all time points investigated (Figure 2A). This indicates that there is no evidence for the development of frank diabetes with long-term Western or high fat feeding. Consistent with similar weight gain between the two elevated fat diet groups, plasma NEFA levels were elevated to a similar extent with Western and high fat diets (Figure 2B). With Western diet, there was a 99% increase in plasma NEFA in the acute term (P<0.01), which was sustained through the intermediate term, such that the average increase in the supply of NEFA to the heart was 116%. With the high fat diet, there was a 182% increase in the acute term (P<0.001) that was maintained throughout the feeding protocol.
Figure 2. Fed plasma NEFA concentrations are elevated in the acute to chronic stages of Western or high fat feeding with no net accumulation of myocardial triacylglycerols or protein carbonylation content.
Fed plasma glucose concentration (A), plasma NEFA concentration (B), myocardial triacylglycerol content (C) and myocardial carbonylated protein content (D) after 1 day and 1 week (acute term, AT), 4 and 8 weeks (short term, ST), 16 and 24 weeks (intermediate term, IT), and 32 and 48 weeks (long term, LT) on low fat, Western or high fat diet. Values are means±S.E.M. for 12–27 independent experiments per group. Closed squares (■) represent low fat diet-fed rats, closed circles (●) represent Western diet-fed rats and closed triangles (▲) represent high fat-fed rats. *, P<0.05, **, P<0.01 and ***, P<0.001 compared with low fat diet at the same age.
To examine whether increased fatty acid supply (i.e. plasma NEFA) resulted in an accumulation of cardiac triacylglycerols, lipids were extracted from hearts. There was no significant increase in cardiac triacylglycerol levels at any time point investigated (Figure 2C); however, there was a trend (P=0.07) for increased myocardial triacylglycerols in the intermediate term with either Western (+46%) or high fat (+42%) diet.
To determine the oxidative stress in the heart due to Western or high fat diet, protein carbonylation content was determined as an indirect marker of oxidative damage. Neither Western nor high fat diet had an effect on protein carbonyl content for the entire feeding protocol (Figure 2D).
Cardiac function is decreased with Western diet in the long term
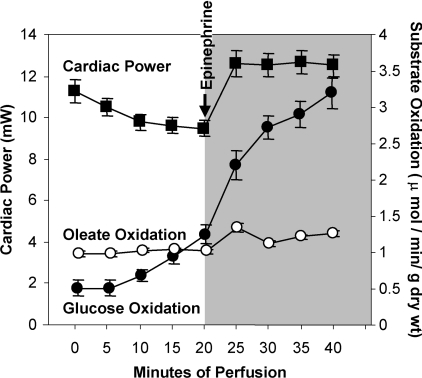
Figure 3 represents the average cardiac power and substrate oxidation for hearts isolated from rats on the low fat diet. At baseline, oleate oxidation predominates as an energy source. With adrenergic stimulation and concomitant increase in afterload, there is a rapid and robust increase in glucose oxidation.
Figure 3. Glucose oxidation flux is increased in response to an acute increase in work in the isolated perfused heart of the Wistar rat on low fat diet.
Representative means±S.E.M. for cardiac power (closed squares, ■), oleate oxidation flux (open circles, ○) and glucose oxidation flux (closed circles, ●) of the Wistar rat on low fat diet (n=58) at baseline (perfusion time 0–20 min) and in response to an acute increase in work (shaded area, perfusion time 25–40 min). Values for subsequent heart perfusion data are presented as the representative value at 20 min of perfusion for baseline measures and at 40 min for measures at increased workload.
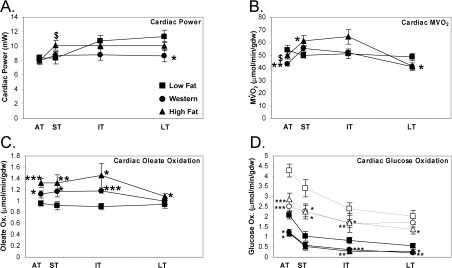
At baseline, there was a maturation of cardiac function over time in the low fat diet group (which is likely attributed to an increase in cardiac size with age; Figure 4A). With high fat diet, there was a similar maturation in cardiac function. However, in comparison with Western diet-fed rats, hearts isolated from high fat-fed rats exhibited increased cardiac power in the short term (P<0.05) (Figure 4A). Furthermore, there was a 25% decrease in cardiac function with Western diet in the long term (P<0.05). With acute adrenergic stimulation and increase in afterload, there was no significant difference in cardiac power for hearts isolated from the three groups (results not shown), suggesting that contractile reserve is maintained with Western and high fat feeding, even in the long term.
Figure 4. Cardiac power is decreased with Western diet in the long term.
Cardiac power (A), MV˙O2 (B), oleate oxidation (C) and glucose oxidation (D) at baseline (solid circles) and in response to an acute increase in work (open points) in the acute (AT), short (ST), intermediate (IT), and long term (LT) on low fat, Western or high fat diet. Values are means±S.E.M. for 12–18 independent experiments per group. Flux is represented per minute per gram dry weight (gdw). Closed squares (■) represent low fat diet-fed rats, closed circles (●) represent Western diet-fed rats and closed triangles (▲) represent high fat-fed rats at baseline. Open squares (□) represent low fat diet-fed rats, open circles (○) represent Western diet-fed rats and open triangles (△) represent high fat diet-fed rats after an acute increase in work. *, P<0.05, **, P<0.01 and ***, P<0.001 compared with low fat diet at the same age. $, P<0.05 compared with Western diet at the same age.
Initially, there was a 21% decrease in MV˙O2 with the Western diet in the acute term (P<0.01) (Figure 4B); MV˙O2 was also 14% lower in Western-diet fed rats than in rats fed a high fat diet at this time. However, there was a trend for an overall increase in MV˙O2 in the hearts of rats fed on the high fat diet compared with the low fat diet in the short (P<0.05) and intermediate (P=0.085) term. In the long term, there was a 15% decrease in oxygen consumption with the Western diet coincident with decreased myocardial function at this time. MV˙O2 increased in all groups with an acute increase in work (results not shown). When an increase in MV˙O2 was observed at baseline for hearts isolated from Western or high fat diet-fed rats, that increase was sustained at the higher workload. A measure of cardiac efficiency (contractile work divided by MV˙O2) was unchanged in hearts of rats fed on Western diet at all time points investigated, but cardiac efficiency was decreased in the intermediate term (−27%, P<0.01) for high fat diet-fed rats (results not shown).
Myocardial oleate oxidation adapts rapidly to an increase in substrate supply. Oleate oxidation was increased in the acute (+16%), short (+28%) and intermediate (+30%) term with the Western diet, but was not increased in the long term (Figure 4C). Oleate oxidation was increased further with the high fat diet in the acute (+38%), short (+44%), intermediate (+62%) and long (+15%) term compared with the low fat diet (Figure 4C). With both diets, the increase in oleate oxidation was sustained with an acute increase in work (results not shown).
Consistent with an increase in fatty acid oxidation, glucose oxidation was suppressed in the hearts of rats fed on a Western or high fat diet (Figure 4D). There was an immediate decrease in the baseline glucose oxidation flux in the short term with Western (−41%) and high fat (−43%) diet in the acute term (P<0.05; Figure 4D); this decrease was seen throughout the feeding protocol. When the hearts were subjected to an acute increase in work, glucose oxidation flux did not increase to the same extent in hearts from Western and high fat diet-fed rats. The findings suggest decreased ‘metabolic flexibility’ of the heart with Western or high fat diet.
Fatty acid-responsive genes involved in fatty acid-mediated futile cycling are differentially induced in the heart by Western and high fat diets
In an attempt to explain the substrate oxidation changes with Western and high fat diets, transcript levels of key metabolic genes involved in fat and glucose utilization were measured. The transcription of pparα was unchanged with Western diet, but decreased in the short (−20%, P<0.05) and intermediate (−23%, P<0.01) term with high fat diet (Figure 5A), which suggests that the increase in PPARα-regulated gene transcripts occurs as a result of ligand activation of PPARα. This decrease in pparα transcription may be a regulatory event to decrease the overall activation of fatty acid utilization when PPARα is chronically activated [10,33].
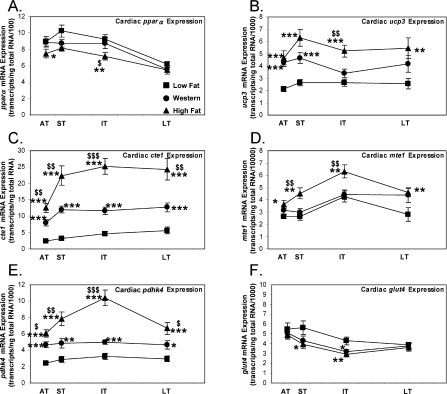
Figure 5. Cardiac expression of fatty acid-responsive genes involved in fatty acid futile cycling are increased to a greater extent by high fat diet compared with Western diet feeding.
mRNA expression of pparα (A), ucp3 (B), cte1 (C), mte1 (D), pdhk4 (E) and glut4 (F) in the hearts of rats fed on low fat, Western or high fat diet in the acute (AT), short (ST), intermediate (IT) and long term (LT). Values are means±S.E.M. for 12–16 independent experiments per group. Closed squares (■) represent low fat diet-fed rats, closed circles (●) represent Western diet-fed rats and closed triangles (▲) represent high fat diet-fed rats. *, P<0.05, **, P<0.01 and ***, P<0.001 versus low fat at the same age. $, P<0.05, $$, P<0.01 and $$$, P<0.001 compared with Western at the same age.
PPARα-regulated genes involved in fatty acid-mediated futile cycling were increased with Western or high fat diet. The transcript levels of UCP3 (uncoupling protein 3) were increased acutely (+102%, P<0.001) with Western diet and remained elevated for the remainder of the study (average +68%; Figure 5B). ucp3 mRNA transcript levels were increased with the high fat diet initially (+119%, P<0.001) and through the long term (average +117%). Compared with the Western diet, the high fat diet increased ucp3 transcripts to a greater extent in the intermediate term (+53%, P<0.01; Figure 5B). The functional significance of an increase in ucp3 mRNA expression in the heart with PPARα activation is not clear. PPARα activation has been shown to increase ucp3 transcript levels and palmitate export from the mitochondrion despite no increase in UCP3 protein levels [34].
We also examined the expression of thioesterases, which hydrolyse fatty acyl-CoAs. CTE1 (cytosolic thioesterase 1) mRNA levels were increased acutely (+226%, P<0.001) with Western diet and remained elevated for the remainder of the feeding protocol (average +196%; Figure 5C). cte1 expression was greatest for hearts isolated from high fat diet-fed rats compared with either low fat (average +451%) or Western diets (average +88%) (Figure 5C). MTE1 (mitochondrial thioesterase 1) mRNA expression was not increased with Western diet. It was, however, increased with high fat diet acutely (+37%, P<0.05) and through the long term (average +55%). The expression of mte1 was greater in the hearts of rats fed on the high fat diet in the short (+51%, P<0.01) and intermediate (+42%, P<0.01) term compared with the Western diet-fed rats (Figure 5D). An increase in mte1 mRNA expression in the heart with diabetes or fenofibrate treatment has been shown to correlate with an increase in MTE1 protein levels and MTE1 activity [34]. Consistent with the observation that only a cassette of genes involved in futile cycling are elevated with Western and high fat diet, mRNA transcript levels of medium-chain acyl dehydrogenase, muscle type CPT (carnitine palmitoyltransferase), CD36 and malonyl-CoA decarboxylase were unchanged with Western or high fat diet at all time points investigated (results not shown).
Regulation of glucose oxidation by the pyruvate dehydrogenase complex is modulated by PPARα-mediated induction of PDHK4 (pyruvate dehydrogenase kinase 4) [10,35,36]. pdhk4 expression in the heart was increased both acutely and at all other time points with the Western diet (average +67%). Interestingly, there was a further activation of pdhk4 expression, when compared with Western diet, with the high fat diet with maximal expression in the intermediate term. Compared with low fat diet, there was an increase in expression of pdhk4 with high fat diet in the acute (+148%, P<0.001), short (+171%, P<0.001), intermediate (+220%, P<0.001) and long (+125%, P<0.001) term (Figure 5E). There was a marked similarity between the activation of pdhk4 expression and activation of oleate oxidation with Western and high fat diets (compare Figure 4C with Figure 5E). Additional regulatory proteins of glucose utilization were altered with Western and high fat diets. The transcription of the insulin-sensitive GLUT4 (glucose transporter 4) was decreased with Western diet in the intermediate term (−26%, P<0.05) and high fat diet in the short (−31%, P<0.05) and intermediate (−32%, P<0.01) term (Figure 5F).
Soleus muscle oleate oxidation maladapts earlier than cardiac oleate oxidation with Western and high fat diets
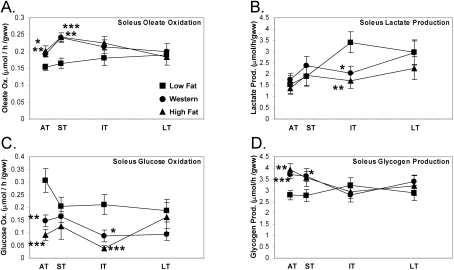
In addition to heart muscle, we also determined insulin-stimulated flux of oleate and glucose oxidation, as well as lactate and glycogen synthesis, in isolated soleus muscles. Our aim was to define the chronology of adaptation and maladaptation to a high fat environment in an oxidative skeletal (soleus) muscle (predominantly type I fibres). Oleate oxidation in soleus muscle was increased initially in the acute and short term with Western or high fat diet (P<0.05; Figure 6A). Interestingly, oleate oxidation flux returned to the flux measured in low fat-fed animals in the intermediate term. This maladaptation was similar to that observed in cardiac muscle, but at an accelerated rate (compare Figure 6A with Figure 4C). Radioactive lactate release in response to insulin stimulation was only decreased in the intermediate term with either Western or high fat diet (Figure 6B), suggesting that there was adequate glucose uptake until the maladaptive decrease in oleate oxidation in the intermediate term. Glucose oxidation was, however, decreased in the acute term (−52%, P<0.01) with Western diet and remained decreased throughout the feeding protocol (average −45%). Similarly, glucose oxidation was decreased in the acute term (−70%, P<0.001) with the high fat diet and remained low in the intermediate term (−82%, P<0.001; Figure 6C). Maximal glycogen synthesis (in response to supra-physiological insulin concentration, 1000 μU/ml) was increased with the Western diet in the acute (+32%, P<0.001) and short (+31%, P<0.05) term (Figure 6D). With high fat diet, glycogen production was only increased in the acute term (+41%, P<0.01). This suggests that, in the early phase of Western and high fat feeding, glucose entry is preserved and, in the face of decreased glucose oxidation, glycogen production is increased.
Figure 6. Soleus muscle oleate oxidation maladapts earlier than cardiac oleate oxidation with Western or high fat diet.
Isolated soleus oleate oxidation (A), radioactive lactate release (B), glucose oxidation flux (C) stimulated with 100 μU/ml insulin, and maximal glycogen production flux (D) stimulated with 1000 μU/ml insulin in the acute (AT), short (ST), intermediate (IT) and long term (LT) on low fat, Western or high fat diet. Values are means±S.E.M. for 12–16 independent experiments per group. Flux is represented per minute per gram wet weight (gww). Closed squares (■) represent low fat diet-fed rats, closed circles (●) represent Western diet-fed rats and closed triangles (▲) represent high fat diet-fed rats. *, P<0.05, **, P<0.01 and ***, P<0.001 compared with low fat diet at the same age.
Diet-induced soleus muscle induction of fatty acid-responsive genes exhibits a similar pattern to cardiac-muscle PPARα activation
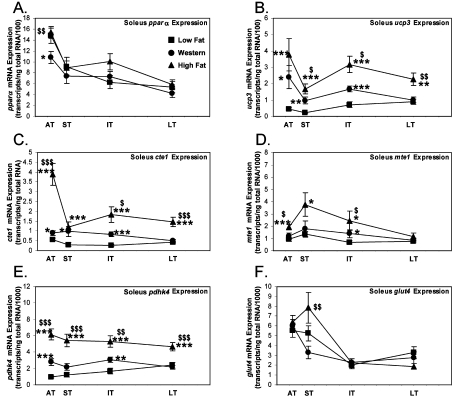
We next sought to determine if the transcriptional changes of PPARα activation seen in cardiac muscle also occur in skeletal muscle. The transcription of pparα decreased only in the acute term (−27%, P<0.05) with Western diet, and was unchanged with the high fat diet (Figure 7A). Fatty acid-responsive genes involved in fatty acid-mediated futile cycling were increased with either Western or high fat diet. The transcription of ucp3 was increased acutely (+433%, P<0.05) with Western diet and remained elevated for the remainder of the study (average +228%; Figure 7B). ucp3 transcription was increased with the high fat diet initially (+748%, P<0.001) and through the long term (average +474%). With high fat diet, the transcription of ucp3 was induced to the greatest extent, compared with the Western diet, in the short (+73%, P<0.05), intermediate (+89%, P<0.05) and long (+127%, P<0.01) term (Figure 7B). cte1 mRNA was increased acutely (+57%, P<0.05) with Western diet and remained elevated in the short (+220%, P<0.05) and intermediate (+221%, P<0.001) term (Figure 7C). cte1 was further increased with the high fat diet compared with either low fat (average +434%) or Western (average +167%) diet (Figure 7C). mte1 expression was increased with the Western diet only in the intermediate term (P<0.05). mte1 transcript levels were, however, increased with high fat diet in the acute term (+107%, P<0.001) and through the intermediate term (average +185%). The expression of mte1 was greater in soleus of rats fed on the high fat diet in the short (+62%, P<0.05) and intermediate (+75%, P<0.05) term compared with the Western diet-fed rats (Figure 7D).
Figure 7. Soleus mRNA expression of fatty acid-responsive genes involved in fatty acid futile cycling is increased to a greater extent by high fat diet feeding compared with Western diet feeding.
mRNA expression of pparα (A), ucp3 (B), cte1 (C), mte1 (D), pdhk4 (E) and glut4 (F) in the soleus muscle of rats fed on low fat, Western or high fat diet in the acute (AT), short (ST), intermediate (IT) and long term (LT). Values are means±S.E.M. for 14–24 independent experiments per group. Closed squares (■) represent low fat diet-fed rats, closed circles (●) represent Western diet-fed rats and closed triangles (▲) represent high fat diet-fed rats. *, P<0.05, **, P<0.01 and ***, P<0.001 compared with low fat diet at the same age. $, P<0.05, $$, P<0.01 and $$$, P<0.001 compared with Western diet at the same age.
pdhk4 expression was increased in only the acute (+188%, P<0.001) and intermediate (+80%, P<0.01) term with the Western diet (Figure 7E). Interestingly, there was further activation of pdhk4 expression, compared with Western diet, with the high fat diet. Compared with low fat diet, there was an increase in expression of pdhk4 in the acute (+539%, P<0.001), short (+343%, P<0.001), intermediate (+213%, P<0.001) and long (+94%, P<0.001) term (Figure 7E). Again, like in heart muscle, there was a marked similarity between the activation of pdhk4 expression and activation of oleate oxidation with Western and high fat diet in soleus muscle (compare Figure 6A with Figure 7E). Soleus glut4 expression was essentially unchanged with Western or high fat diets (Figure 7F).
DISCUSSION
The main findings of our study are as follows: (i) Wistar rats fed on a Western diet develop cardiac dysfunction after 8–12 months on the diet. This dysfunction is not observed in rats fed on the high fat diet. (ii) With Western diet, there is an activation of oleate oxidation and an increase in the mRNA transcripts of specific genes involved in fatty acid-mediated futile cycling, although to a lesser extent as measured in hearts of rats fed on the high fat diet. These observations suggest a potentially inadequate activation of uncoupling, which may contribute to the development of contractile dysfunction with the Western diet (Figure 8). (iii) In skeletal muscle, when compared with the heart, there is a similar adaptation of substrate oxidation and transcription of fatty acid-responsive genes with Western or high fat diet. These processes maladapt at an accelerated rate in skeletal muscle, and could expound lipotoxicity developed in the heart.
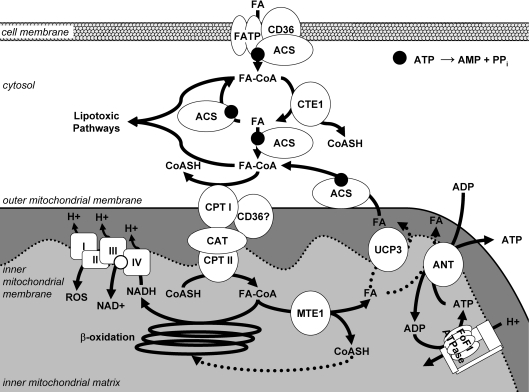
Figure 8. Putative role of the decrease in fatty acid-responsive genes in the development of contractile dysfunction with Western diet compared with high fat diet feeding.
Fatty acid (FA) enters the cell through fatty acid transporters, fatty acid transport protein (FATP) and or fatty acid translocase (CD36), and is activated to fatty acyl-CoA (FA-CoA) by an isoform of acyl-CoA synthetase (ACS). The activated FA-CoA can undergo hydrolysis (at the indirect expense of ATP) via CTE1, which is decreased in the Western diet compared with the high fat diet, thus decreasing the capacity for fatty acid-mediated futile cycling. After entry of FA-CoAs into the mitochondrion via CPT and carnitine acyltransferase (CAT), there is a decrease in the capacity of MTE1 and UCP3 to ‘uncouple’ fat oxidation (indicated with dashed lines) with the Western diet compared with the high fat diet, which may result in a decreased free CoASH pool for complete β-oxidation and increased production of ROS by the electron transport chain (ETC). Therefore FA-CoAs accumulate and are shuttled into ‘lipotoxic pathways’.
We have identified inadequate activation of fatty acid-responsive genes as a potential mechanism for the development of contractile dysfunction with Western diet. Because decreased activation of futile cycling components was only seen with Western diet, this implicates inadequate activation of PPARα (and/or PPARβ/δ) or a co-activator of PPARα as a consequence of glucolipotoxicity [37]. We found that supply of increased saturated fatty acids in the diet with the high fat diet compared with the Western diet (Table 1) promoted higher expression of a select cassette of fatty acid-responsive genes. In addition to the relative decrease in fatty acid supply, the Western diet also contains a relative increase in carbohydrates (15% of total calories) compared with the high fat diet. Previous reports have found that oversupply of glucose can inhibit PPARα expression/activity as well as target gene expression [33,38].
We have identified suboptimal induction of fatty acid-responsive genes to occur as rapidly as the acute phase of Western diet feeding, suggesting that PPARα or its co-activators/co-repressors are modified after 1 day of feeding on the Western diet. Potential mechanisms include phosphorylation of PPARα itself [39], or other co-activators, or the regulation of transcriptional modulators through additional mechanisms such as modification with small ubiquitin-related modifiers [40]. The long-term consequences of inadequate activation of fatty acid oxidation in the face of high fat exposure may culminate in contractile dysfunction (Figure 8). These findings are consistent with previous reports that obese Zucker rat hearts are unable to respond to increased fatty acid availability, resulting in contractile dysfunction [19]. However, the question remains which end processes of PPARα activation are adaptive (i.e. alleviate lipotoxicity) or are maladaptive (i.e. worsen lipotoxicity).
As with deposition of neutral triacylglycerol in the heart, there is debate whether futile cycling/uncoupling is adaptive or deleterious to cardiac contractile function. ROS (reactive oxygen species) generation is deleterious to numerous cellular functions and is thought to promote fatty acid uncoupling [41–43]. Diabetes is known to increase ROS and uncoupling in the heart, but it is felt that uncoupling may also be deleterious to overall function and may explain the decreased cardiac efficiency that is measured in diabetes [44]. Our work points to uncoupling as a beneficial adaptation, potentially through alleviation of ROS generation. We find preservation of contractile function with the high fat diet, when induction of fatty acid-mediated futile cycle components occurs to a greater extent. In addition, there is much speculation on the role and importance of UCP3 in heart and skeletal-muscle tissue [45]. While the exact mechanism of UCP3 remains unknown, the up-regulation of UCP3 during a high fat stress provides clues to the involvement of UCP3 in dissipating energy during states of excess, potentially harmful, oxidative phosphorylation of lipids (Figure 8).
In the absence of direct measures of food consumption or whole body energy expenditure, rats on the Western diet gained a similar amount of weight compared with those on the high fat diet despite the fact that the animals on the high fat diet ate an estimated 11% more calories per day (C. R. Wilson and H. Taegtmeyer, unpublished work). Assuming that the rats in both groups had the same amount of activity, this suggests that, on a whole body level, rats on the high fat diet had a higher metabolic rate, which supports greater activation of uncoupling processes in multiple tissues including the heart and skeletal muscle.
In a study this large, there are also many limitations. Foremost, we characterized cardiac function in the ex vivo model so that we could minimize acute neurohumoral influences that may compensate for inherent alterations in cardiac performance. Because we maintained a substrate composition for all groups that was similar to the low fat-fed rat milieu, it is possible that the dysfunction we measured in the Western or high fat-fed rat hearts may be due to inadequate supply of fatty acids in the perfusate. We did not measure the products of incomplete oxidation of fatty acids (i.e. acylcarnitine derivatives) in our model. Previous work in rat skeletal muscle has shown accumulation of acylcarnitines in the transition of fasted to fed state in rats fed on a high fat diet [46]. Also, we were not able to obtain direct measurements of glucose or fatty acid uptake in the heart or skeletal muscle. Instead, we chose a radioactive tracer strategy designed to simultaneously measure glucose and oleate oxidation flux, which we considered necessary to define adaptation and maladaptation to the high fat environment. Glucose and fatty acid uptake would be very useful measures in the present study, but these measures would have required an additional set of heart perfusions and soleus muscle incubations. Although we did not observe a significant difference in myocardial triacylglycerol levels between the feeding groups, it is possible that additional lipotoxic intermediates (e.g. ceramide) are altered. Lastly, the present study has not attempted an intervention to assess the possible reversibility of the remodelling and dysfunction that was observed with the Western diet. Other groups have demonstrated in the rat model reversibility of plasma lipid abnormalities and vascular function induced by 8 weeks of high fat diet after 8 weeks of normal chow feeding [47]. Also, many changes in substrate oxidation and transcription occurred after only 1 day on the diet, raising the possibility that these parameters may be readily reversible. Whether cardiac dysfunction due to long-term exposure to inadequate activation of fatty acid-utilizing pathways is reversible requires further investigation.
Conclusions
Wistar rats fed on a Western diet develop cardiac dysfunction ex vivo, while function is maintained in rats fed on the high fat diet. The dysfunction occurs in the setting of prolonged suboptimal transcriptional activation of fatty acid-responsive genes involved in futile cycling and regulation of pyruvate oxidation and in the submaximal activation of fat oxidation. We implicate the prolonged exposure to these maladaptive responses in the development of contractile dysfunction with Western diet.
Acknowledgments
We thank Patrick H. Guthrie for technical assistance. We also thank Terri M. King for help with the data analysis. The research was supported, in part, by grants from the National Heart, Lung and Blood Institute (RO1-HL073162 to H. T. and HL074259 to M. E. Y.). C. R. W. received additional support from the Harry S. and Isabel C. Cameron Foundation. M. K. T. received an institutional training grant from the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) (T35 DK007676).
References
- 1.Taegtmeyer H., McNulty P., Young M. E. Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 2.Ogden C. L., Carroll M. D., Curtin L. R., McDowell M. A., Tabak C. J., Flegal K. M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA, J. Am. Med. Assoc. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S., Evans J. C., Levy D., Wilson P. W., Benjamin E. J., Larson M. G., Kannel W. B., Vasan R. S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 4.Peterson L. R., Waggoner A. D., Schechtman K. B., Meyer T., Gropler R. J., Barzilai B., Davila-Roman V. G. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J. Am. Coll. Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D., Ascherio A., Hu F. B., Stampfer M. J., Willett W. C., Siscovick D. S., Rimm E. B. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–164. doi: 10.1161/01.CIR.0000152099.87287.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozaffarian D., Katan M. B., Ascherio A., Stampfer M. J., Willett W. C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 8.Barger P. M., Kelly D. P. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc. Med. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 9.Finck B. N., Han X., Courtois M., Aimond F., Nerbonne J. M., Kovacs A., Gross R. W., Kelly D. P. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young M. E., Patil S., Ying J., Depre C., Ahuja H. S., Shipley G. L., Stepkowski S. M., Davies P. J., Taegtmeyer H. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB J. 2001;15:833–845. doi: 10.1096/fj.00-0351com. [DOI] [PubMed] [Google Scholar]
- 11.Boudina S., Sena S., O'Neill B. T., Tathireddy P., Young M. E., Abel E. D. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 12.Durgan D. J., Smith J. K., Hotze M. A., Egbejimi O., Cuthbert K. D., Zaha V. G., Dyck J. R., Abel E. D., Young M. E. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2480–H2497. doi: 10.1152/ajpheart.01344.2005. [DOI] [PubMed] [Google Scholar]
- 13.Stavinoha M. A., RaySpellicy J. W., Essop M. F., Graveleau C., Abel E. D., Hart-Sailors M. L., Mersmann H. J., Bray M. S., Young M. E. Evidence for mitochondrial thioesterase 1 as a peroxisome proliferator-activated receptor-alpha-regulated gene in cardiac and skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004;287:E888–E895. doi: 10.1152/ajpendo.00190.2004. [DOI] [PubMed] [Google Scholar]
- 14.Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 15.Taegtmeyer H., Wilson C. R., Razeghi P., Sharma S. Metabolic energetics and genetics in the heart. Ann. N.Y. Acad. Sci. 2005;1047:208–218. doi: 10.1196/annals.1341.019. [DOI] [PubMed] [Google Scholar]
- 16.Tian R. Transcriptional regulation of energy substrate metabolism in normal and hypertrophied heart. Curr. Hypertens. Rep. 2003;5:454–458. doi: 10.1007/s11906-003-0052-7. [DOI] [PubMed] [Google Scholar]
- 17.Hansen P. A., Han D. H., Marshall B. A., Nolte L. A., Chen M. M., Mueckler M., Holloszy J. O. A high fat diet impairs stimulation of glucose transport in muscle. Functional evaluation of potential mechanisms. J. Biol. Chem. 1998;273:26157–26163. doi: 10.1074/jbc.273.40.26157. [DOI] [PubMed] [Google Scholar]
- 18.Park S. Y., Cho Y. R., Kim H. J., Higashimori T., Danton C., Lee M. K., Dey A., Rothermel B., Kim Y. B., Kalinowski A., et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 19.Young M. E., Guthrie P. H., Razeghi P., Leighton B., Abbasi S., Patil S., Youker K. A., Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 20.Mazumder P. K., O'Neill B. T., Roberts M. W., Buchanan J., Yun U. J., Cooksey R. C., Boudina S., Abel E. D. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan J., Mazumder P. K., Hu P., Chakrabarti G., Roberts M. W., Yun U. J., Cooksey R. C., Litwin S. E., Abel E. D. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 22.Taegtmeyer H., Hems R., Krebs H. A. Utilization of energy-providing substrates in the isolated working rat heart. Biochem. J. 1980;186:701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin G. W., Taylor C. S., Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J. Biol. Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 24.Krebs H. A., Henseleit K. Untersuchungen über die Harnstoffbildung im Tierkörper. Hoppe-Seyler's Z. Physiol. Chem. 1932;210:33–66. [Google Scholar]
- 25.Young M. E., Radda G. K., Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem. J. 1997;322:223–228. doi: 10.1042/bj3220223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Depre C., Shipley G. L., Chen W., Han Q., Doenst T., Moore M. L., Stepkowski S., Davies P. J., Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat. Med. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 27.Young M. E., Razeghi P., Cedars A. M., Guthrie P. H., Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ. Res. 2001;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 28.Stavinoha M. A., RaySpellicy J. W., Hart-Sailors M. L., Mersmann H. J., Bray M. S., Young M. E. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am. J. Physiol. Endocrinol. Metab. 2004;287:E878–E887. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 29.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 30.Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A. G., Ahn B. W., Shaltiel S., Stadtman E. R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S., Dewald O., Adrogue J., Salazar R. L., Razeghi P., Crapo J. D., Bowler R. P., Entman M. L., Taegtmeyer H. Induction of antioxidant gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species. Free Radical Biol. Med. 2006;40:2223–2231. doi: 10.1016/j.freeradbiomed.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Richert S., Wehr N. B., Stadtman E. R., Levine R. L. Assessment of skin carbonyl content as a noninvasive measure of biological age. Arch. Biochem. Biophys. 2002;397:430–432. doi: 10.1006/abbi.2001.2683. [DOI] [PubMed] [Google Scholar]
- 33.Yan J., Young M. E., Lopaschuk G. D., Liao R., Tian R. Substrate availability regulates energy metabolism via transcriptional mechanisms. Circulation. 2006;114(Suppl. II):135. [Google Scholar]
- 34.King K. L., Young M. E., Kerner J., Huang H., O'shea K M., Alexson S. E., Hoppel C. L., Stanley W. C. Diabetes or peroxisome proliferator-activated receptor α agonist increases mitochondrial thioesterase I activity in heart. J. Lipid. 2007;48:1511–1517. doi: 10.1194/jlr.M600364-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Sugden M. C., Holness M. J. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch. Physiol. Biochem. 2006;112:139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- 36.Wu P., Peters J. M., Harris R. A. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor alpha. Biochem. Biophys. Res. Commun. 2001;287:391–396. doi: 10.1006/bbrc.2001.5608. [DOI] [PubMed] [Google Scholar]
- 37.Young M. E., McNulty P., Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 38.Roduit R., Morin J., Masse F., Segall L., Roche E., Newgard C. B., Assimacopoulos-Jeannet F., Prentki M. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-alpha gene in the pancreatic beta-cell. J. Biol. Chem. 2000;275:35799–35806. doi: 10.1074/jbc.M006001200. [DOI] [PubMed] [Google Scholar]
- 39.Barger P. M., Brandt J. M., Leone T. C., Weinheimer C. J., Kelly D. P. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J. Clin. Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi S. J., Chung S. S., Rho E. J., Lee H. W., Lee M. H., Choi H. S., Seol J. H., Baek S. H., Bang O. S., Chung C. H. Negative modulation of RXRalpha transcriptional activity by small ubiquitin-related modifier (SUMO) modification and its reversal by SUMO-specific protease SUSP1. J. Biol. Chem. 2006;281:30669–30677. doi: 10.1074/jbc.M604033200. [DOI] [PubMed] [Google Scholar]
- 41.Cadenas S., Echtay K. S., Harper J. A., Jekabsons M. B., Buckingham J. A., Grau E., Abuin A., Chapman H., Clapham J. C., Brand M. D. The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J. Biol. Chem. 2002;277:2773–2778. doi: 10.1074/jbc.M109736200. [DOI] [PubMed] [Google Scholar]
- 42.Echtay K. S., Roussel D., St-Pierre J., Jekabsons M. B., Cadenas S., Stuart J. A., Harper J. A., Roebuck S. J., Morrison A., Pickering S., et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 43.Korshunov S. S., Korkina O. V., Ruuge E. K., Skulachev V. P., Starkov A. A. Fatty acids as natural uncouplers preventing generation of O2•− and H2O2 by mitochondria in the resting state. FEBS Lett. 1998;435:215–218. doi: 10.1016/s0014-5793(98)01073-4. [DOI] [PubMed] [Google Scholar]
- 44.Boudina S., Abel E. D. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. doi: 10.1152/physiol.00008.2006. [DOI] [PubMed] [Google Scholar]
- 45.Bezaire V., Seifert E. L., Harper M. E. Uncoupling protein-3: clues in an ongoing mitochondrial mystery. FASEB J. 2007;21:312–324. doi: 10.1096/fj.06-6966rev. [DOI] [PubMed] [Google Scholar]
- 46.Koves T. R., Li P., An J., Akimoto T., Slentz D., Ilkayeva O., Dohm G. L., Yan Z., Newgard C. B., Muoio D. M. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 47.Naderali E. K., Fatani S., Williams G. Chronic withdrawal of a high-palatable obesity-inducing diet completely reverses metabolic and vascular abnormalities associated with dietary-obesity in the rat. Atherosclerosis. 2004;172:63–69. doi: 10.1016/j.atherosclerosis.2003.09.021. [DOI] [PubMed] [Google Scholar]