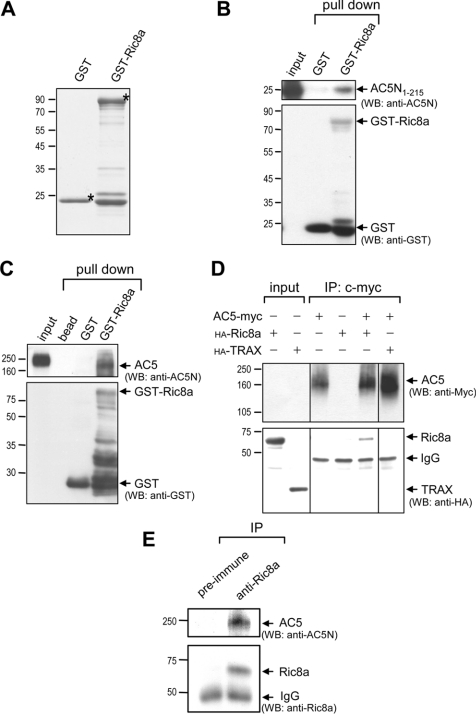
Figure 1. Ric8a interacts with AC5 in vitro.
(A) Purified recombinant GST fusion proteins (2 μg) were resolved by SDS/PAGE (15% gels) and visualized by staining with Coomassie Blue. Asterisks (*) indicate the corresponding recombinant protein of the correct size. The purified recombinant GST fusion protein (20 μg) was incubated with purified AC5N1–215 (15 μg) (B) or the soluble striatal membrane fractions (250 μg) (C) for 60 min at 4 °C to allow complex formation. The bound proteins were separated by SDS/PAGE (10–12% gels), followed by Western blot analyses. GST–Ric8a, but not by GST, effectively pulled-down AC5N1–215 (B) and AC5 harvested from the rat striatum (C). (D) HEK-293T cells were harvested 48 h after transfection with the indicated cDNAs for immunoprecipitation followed by Western blot analyses using the indicated antibodies. (E) Immunoprecipitation of endogenous Ric8a using the anti-Ric8a antibody from the striatum successfully pulled-down striatal AC5 detected using the anti-AC5N antibody. TRAX, translin-associated protein X.