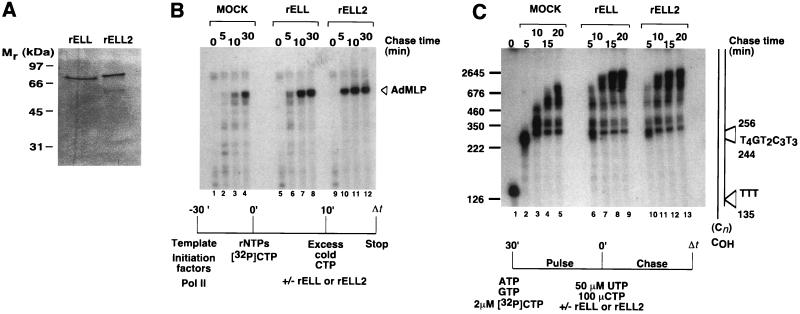
Figure 3.
ELL2 and ELL have similar effects on elongation by RNA polymerase II during synthesis of promoter-independent and promoter-dependent transcripts. (A) Ten percent SDS/PAGE of recombinant ELL2 (rELL2) and ELL (rELL), purified by nickel chromatography and preparative SDS/PAGE. Proteins were visualized by silver staining. (B) Effects of ELL2 and ELL on the kinetics of promoter-dependent transcription. Preinitiation complexes were assembled at the AdML promoter with recombinant TBP, TFIIB, TFIIE, TFIIF, and purified rat TFIIH and RNA polymerase II as described (13). Transcription was initiated by addition of 50 μM ATP, 50 μM GTP, 2 μM UTP, 10 μCi of [α-32P]CTP (>400 Ci/mmol, Amersham), and 7 mM MgCl2. After 10 min at 28°C, 100 μM nonradioactive CTP was added to reaction mixture and short transcripts were chased in the absence or presence of ≈50 ng of SDS/PAGE-purified rELL2 or rELL for the times indicated. Transcripts were analyzed by electrophoresis through a 6% polyacrylamide/7.0 M urea gel. (C) Effects of ELL2 and ELL on the kinetics of promoter-independent transcription. SDS/PAGE-purified histidine-tagged ELL2 and ELL proteins were renatured and assayed in pulse–chase reactions as diagrammed in the figure using the oligo(dC)-tailed template pCpGR220 S/P/X. Reactions contained ≈0.01 unit of RNA polymerase II, 100 ng of pCpGR220S/P/X, and ≈50 ng of rELL2 or ≈50 ng of ELL and were performed essentially as described (13). The control reaction (mock) contained an identically prepared fraction from uninfected JM109(DE3) cells.