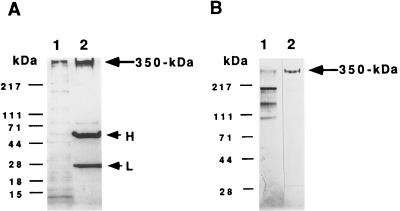
Figure 1.
Isolation of receptor by immunoaffinity chromatography. (A) Immunoprecipitation of S. purpuratus 350-kDa protein. In A, after incubation of the solubilized S. purpuratus egg cell CSC with mAb RS132 bound to protein A-agarose beads, the recovered beads were washed three times with TBS and then treated with SDS sample buffer containing 5% 2-mercaptoethanol and heated at 75°C for 15 min. Samples were then analyzed for bound proteins by 4–15% gradient SDS/PAGE under reducing conditions, followed by silver staining. Lanes: 1, solubilized egg CSC; 2, the proteins bound and eluted from the beads. H and L shows the positions of IgG heavy and light chains, respectively. (B) Analysis of receptor protein purified by immunoaffinity chromatography. For analysis, samples were electrophoresed on a 4–15% gradient SDS/polyacrylamide gel. Lanes: 1, solubilized egg CSC; 2, the fraction eluted with 5 M LiCl/10 mM Tris·Cl buffer (pH 7.4) from an affinity column made by covalently coupling mAb RS132 to protein A-agarose as described (20). Bio-Rad protein molecular markers: myosin (H-chain), 217 kDa; phosphorylase B, 111 kDa; BSA, 71 kDa; ovalbumin, 44 kDa; carbonic anhydrase, 28 kDa; β-lactoglobulin, 18 kDa; and lysozyme, 15 kDa.