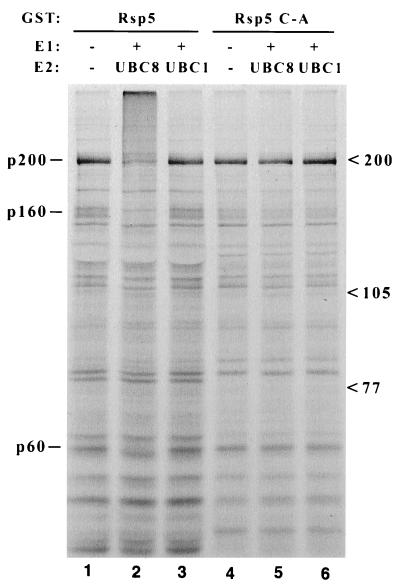
Figure 1.
Binding and ubiquitination of 35S-labeled yeast proteins by Rsp5. Glutathione-Sepharose-bound GST-Rsp5 fusion proteins, either wild type (lanes 1–3) or the C-A mutant (lanes 4–6), were incubated with 35S-labeled total yeast extract. The Sepharose beads were collected, washed, and then incubated without (lanes 1 and 4) or with (lanes 2, 3, 5, 6) recombinant E1 enzyme and UBC8 (lanes 2 and 5) or UBC1 (lanes 3 and 6) E2 protein. Proteins were then denatured in loading buffer and analyzed by SDS/PAGE and autoradiography. Three protein species that appear to be bound and ubiquitinated specifically by wild-type GST-Rsp5/UBC8 are indicated at left (p200, p160, p60), and migration positions of molecular weight markers are indicated at right.