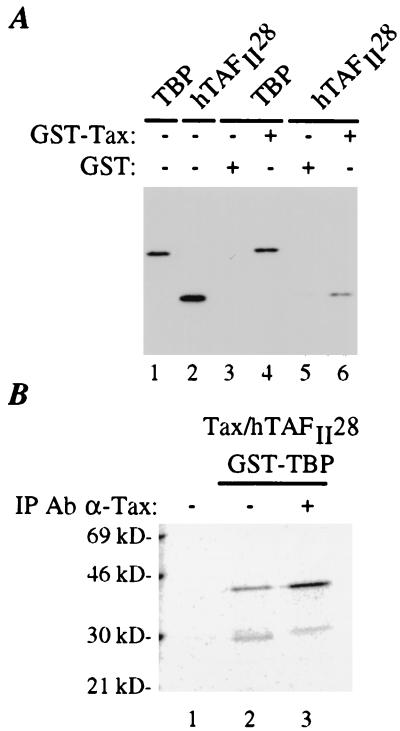
Figure 2.
hTAFII28 interacts in vitro with Tax. (A) The GST and GST-Tax proteins coupled to glutathione-Sepharose beads (7) were incubated with purified TBP (9) (lanes 3 and 4) or purified His-hTAFII28 (10) (lanes 5 and 6). Proteins were uncoupled from the beads by incubation in SDS/PAGE loading buffer for 10 min at 90°C and migrated through a SDS/10% polyacrylamide protein gel. As controls, 5% of the amounts of TBP and hTAFII28 used for the incubations with GST and GST-Tax was loaded directly onto the gel (lanes 1 and 2, respectively). The gel was analyzed by immunoblotting with a mix of mAbs directed against TBP (3G3) and hTAFII28 (15TA + 1C9). (B) Tax and hTAFII28 were produced and labeled with [35S]methionine by in vitro-coupled transcription/translation. These proteins were incubated with the GST-TBP fusion protein coupled to glutathione Sepharose beads. After three washes, the proteins were eluted by treatment with free glutathione. One-tenth of the eluted proteins was loaded onto a SDS protein gel (lane 2). The eluted proteins were next immunoprecipitated with an antibody directed against Tax in Nonidet P-40 lysis buffer (17), and the immunoprecipitated proteins were analyzed by SDS/PAGE (lane 3). The gel was dried and exposed to a Phosphor screen of which image is shown. In lane 1, the radioactively labeled Tax and hTAFII28 proteins were incubated with protein A Sepharose beads to control for nonspecific binding. The positions of the bands of a molecular weight marker run in parallel are indicated.