Abstract
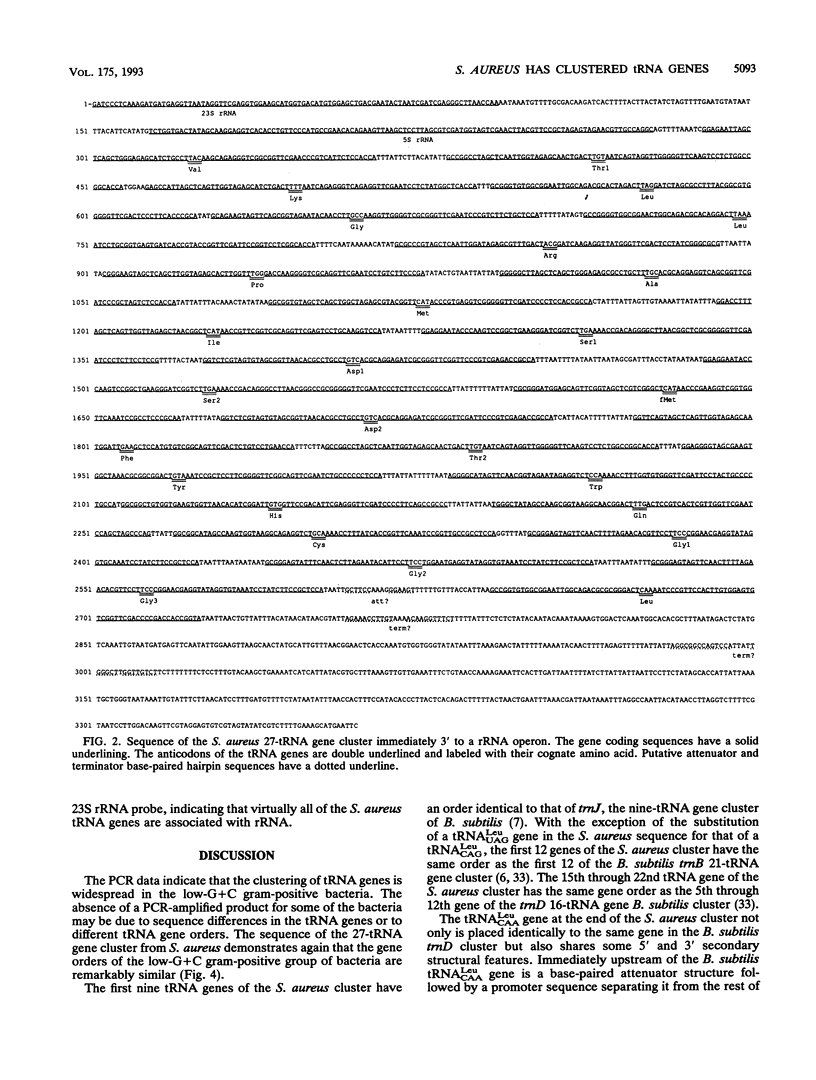
The polymerase chain reaction (PCR) was used to detect large tRNA gene clusters in Bacillus subtilis, Bacillus badius, Bacillus megaterium, Lactobacillus brevis, Lactobacillus casei, and Staphylococcus aureus. The primers were based on conserved sequences of known gram-positive bacterial tRNA(Arg) and tRNA(Phe) genes. This PCR procedure detected an unusually large tRNA gene cluster in S. aureus. PCR-generated probes were used to identify a 4.5-kb EcoRI fragment that contained 27 tRNA genes immediately 3' to an rRNA operon. Some of these 27 tRNA genes are very similar, but only 1 is exactly repeated in the cluster. The 5' end of this cluster has a gene order similar to that found in the 9- and 21-tRNA gene clusters of B. subtilis. The 3' end of this S. aureus cluster exhibits more similarity to the 16-tRNA gene cluster of B. subtilis. The 24th, 25th, and 26th tRNA genes of this S. aureus tRNA gene cluster code for three similar, unusual Gly-tRNAs that may be used in the synthesis of the peptidoglycan in the cell wall but not in protein synthesis. Southern analysis of restriction digests of S. aureus DNA indicate that there are five to six rRNA operons in this bacterium's genome and that most or all may have large tRNA gene clusters at the 3' end.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Bumsted R. M., Dahl J. L., Söll D., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. X. Further study of the glycyl transfer ribonucleic acids active in peptidoglycan synthesis in Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):779–782. [PubMed] [Google Scholar]
- Emilsson V., Kurland C. G. Growth rate dependence of transfer RNA abundance in Escherichia coli. EMBO J. 1990 Dec;9(13):4359–4366. doi: 10.1002/j.1460-2075.1990.tb07885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley L. S., Yarus M. Codon contexts from weakly expressed genes reduce expression in vivo. J Mol Biol. 1989 Oct 5;209(3):359–378. doi: 10.1016/0022-2836(89)90003-x. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Wisotzkey J. D., Jurtshuk P., Jr How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992 Jan;42(1):166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. A cluster of nine tRNA genes between ribosomal gene operons in Bacillus subtilis. J Bacteriol. 1992 May;174(10):3147–3151. doi: 10.1128/jb.174.10.3147-3151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Sequence analysis of a cluster of twenty-one tRNA genes in Bacillus subtilis. Nucleic Acids Res. 1983 Aug 25;11(16):5763–5774. doi: 10.1093/nar/11.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Zinoni F., Sawers G., Mandrand-Berthelot M. A., Böck A. Escherichia coli genes whose products are involved in selenium metabolism. J Bacteriol. 1988 Feb;170(2):540–546. doi: 10.1128/jb.170.2.540-546.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K., Lund E., Dahlberg J. E. tRNA genes are found between 16S and 23S rRNA genes in Bacillus subtilis. Nucleic Acids Res. 1982 Mar 11;10(5):1607–1624. doi: 10.1093/nar/10.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Dietrich C. P., Strominger J. L. Incorporation of glycine into the cell wall glycopeptide in Staphylococcus aureus: role of sRNA and lipid intermediates. Proc Natl Acad Sci U S A. 1965 Aug;54(2):587–594. doi: 10.1073/pnas.54.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N. Markedly unbiased codon usage in Bacillus subtilis. Gene. 1985;40(1):145–150. doi: 10.1016/0378-1119(85)90035-6. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., Yoshikawa H. Structure and organization of rRNA operons in the region of the replication origin of the Bacillus subtilis chromosome. Nucleic Acids Res. 1983 Sep 24;11(18):6301–6318. doi: 10.1093/nar/11.18.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. F., Strominger J. L., Söll D. Biosynthesis of the peptidoglycan of bacterial cell walls. VII. Incorporation of serine and glycine into interpeptide bridges in Staphylococcus epidermidis. J Biol Chem. 1968 Feb 25;243(4):757–767. [PubMed] [Google Scholar]
- Roberts R. J., Lovinger G. G., Tamura T., Strominger J. L. Staphylococcal transfer ribonucleic acids. I. Isolation and purification of the isoaccepting glycine transfer ribonucleic acids from Staphylococcus epidermidis Texas 26. J Biol Chem. 1974 Aug 10;249(15):4781–4786. [PubMed] [Google Scholar]
- Roberts R. J. Staphylococcal transfer ribonucleic acids. II. Sequence analysis of isoaccepting glycine transfer ribonucleic acids IA and IB from Staphylococcus epidermidis Texas 26. J Biol Chem. 1974 Aug 10;249(15):4787–4796. [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. Organization and structure of tRNA genes in Spiroplasma melliferum. Isr J Med Sci. 1987 May;23(5):357–360. [PubMed] [Google Scholar]
- Rowley K. B., Elford R. M., Roberts I., Holmes W. M. In vivo regulatory responses of four Escherichia coli operons which encode leucyl-tRNAs. J Bacteriol. 1993 Mar;175(5):1309–1315. doi: 10.1128/jb.175.5.1309-1315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner R., Chevrestt A., Buchholz S. R., Studamire B., White A. M., Jarvis E. D. Two tRNA gene clusters associated with rRNA operons rrnD and rrnE in Bacillus subtilis. J Bacteriol. 1993 Jan;175(2):503–509. doi: 10.1128/jb.175.2.503-509.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Guindy Y. S., Lustig F., Borén T., Lagerkvist U. Apparent lack of discrimination in the reading of certain codons in Mycoplasma mycoides. Proc Natl Acad Sci U S A. 1987 May;84(10):3166–3170. doi: 10.1073/pnas.84.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpaer E. G. Constraints on codon context in Escherichia coli genes. Their possible role in modulating the efficiency of translation. J Mol Biol. 1986 Apr 20;188(4):555–564. doi: 10.1016/s0022-2836(86)80005-5. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Dank N., Nock S., Schön A. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2127–2171. doi: 10.1093/nar/19.suppl.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Andachi Y., Muto A. Evolution of tRNAs and tRNA genes in Acholeplasma laidlawii. Nucleic Acids Res. 1991 Dec 25;19(24):6787–6792. doi: 10.1093/nar/19.24.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S., Green C. J., Narasimhan N., Strem M., Hansen J. N. Transcriptional analysis of Bacillus subtilis rRNA-tRNA operons. II. Unique properties of an operon containing a minor 5 S rRNA gene. J Biol Chem. 1988 Oct 5;263(28):14485–14490. [PubMed] [Google Scholar]
- Vold B. S., Okamoto K., Murphy B. J., Green C. J. Transcriptional analysis of Bacillus subtilis rRNA-tRNA operons. I. The tRNA gene cluster of rrnB has an internal promoter. J Biol Chem. 1988 Oct 5;263(28):14480–14484. [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Ohki M., Ishikura H. The nucleotide sequence of Bacillus subtilis tRNA genes. Nucleic Acids Res. 1983 May 25;11(10):3037–3045. doi: 10.1093/nar/11.10.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]