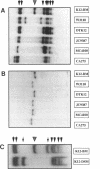
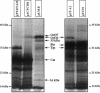
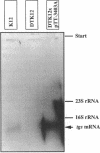
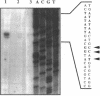
Abstract
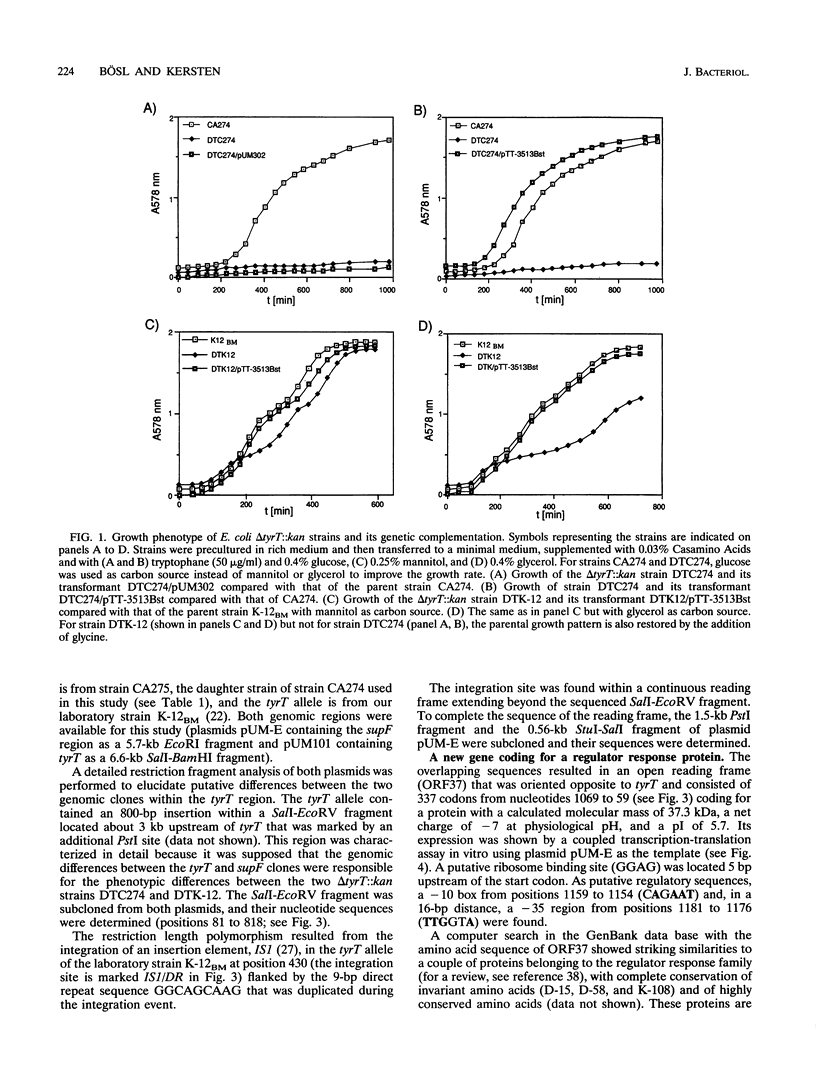
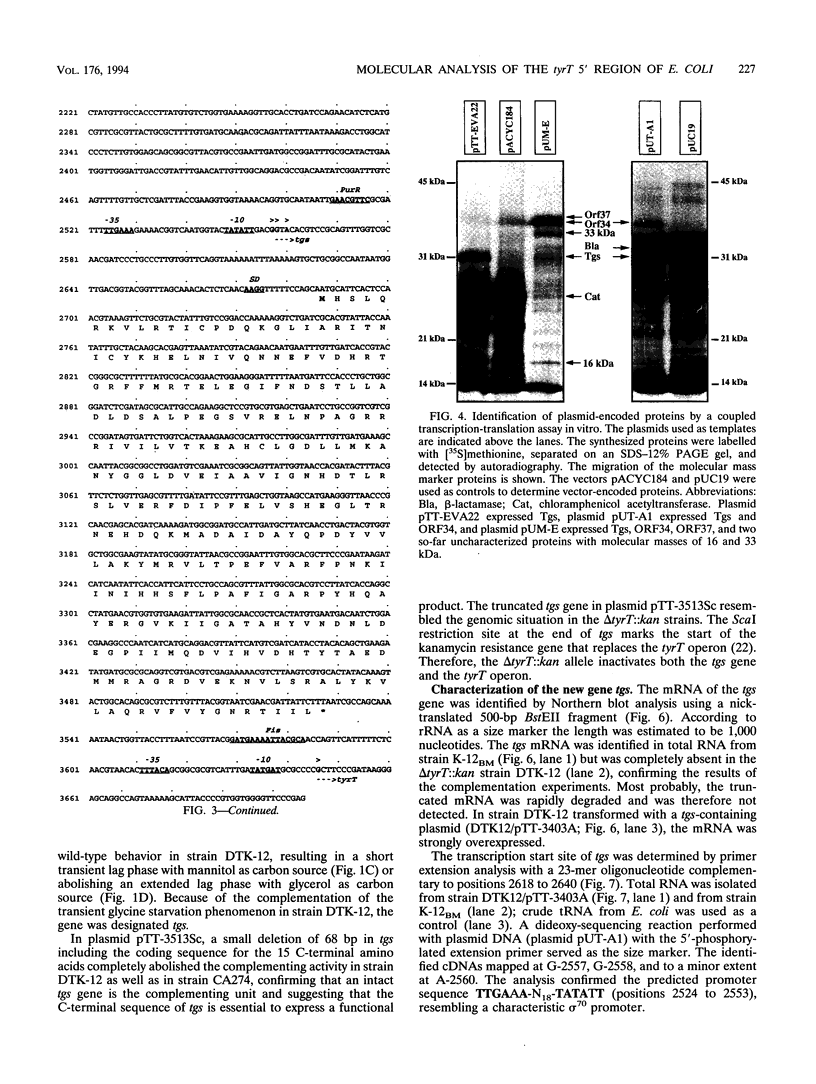
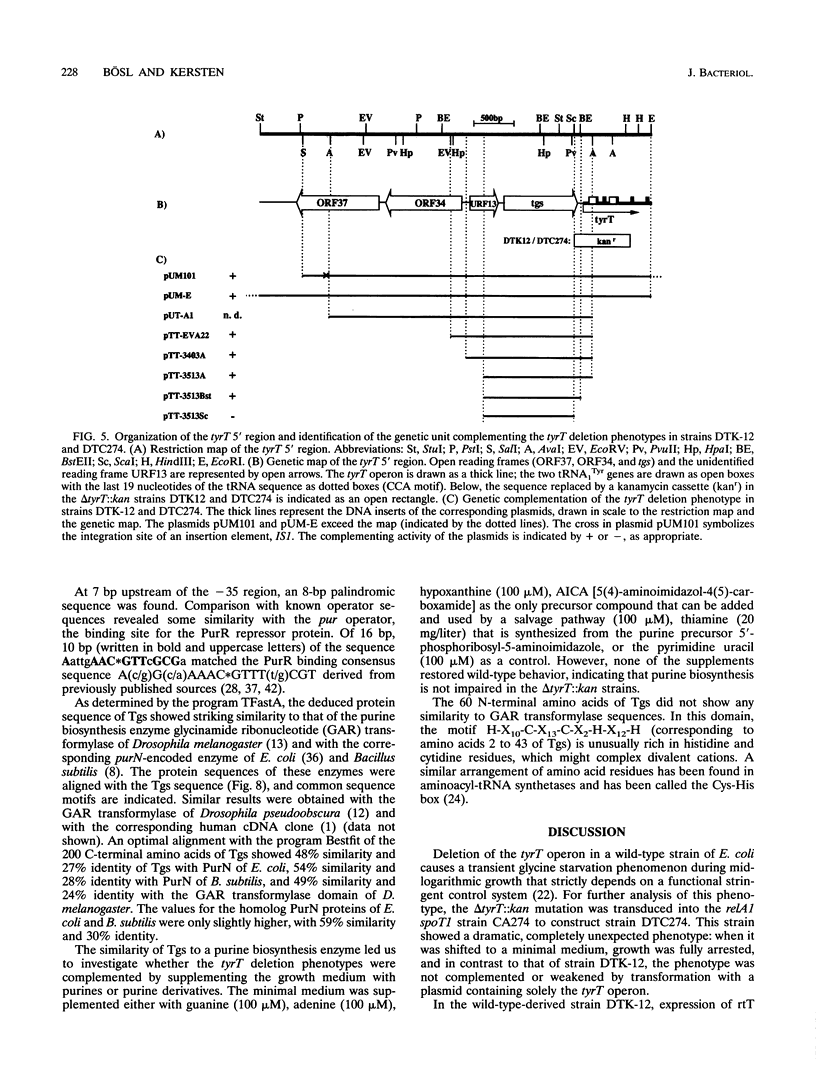
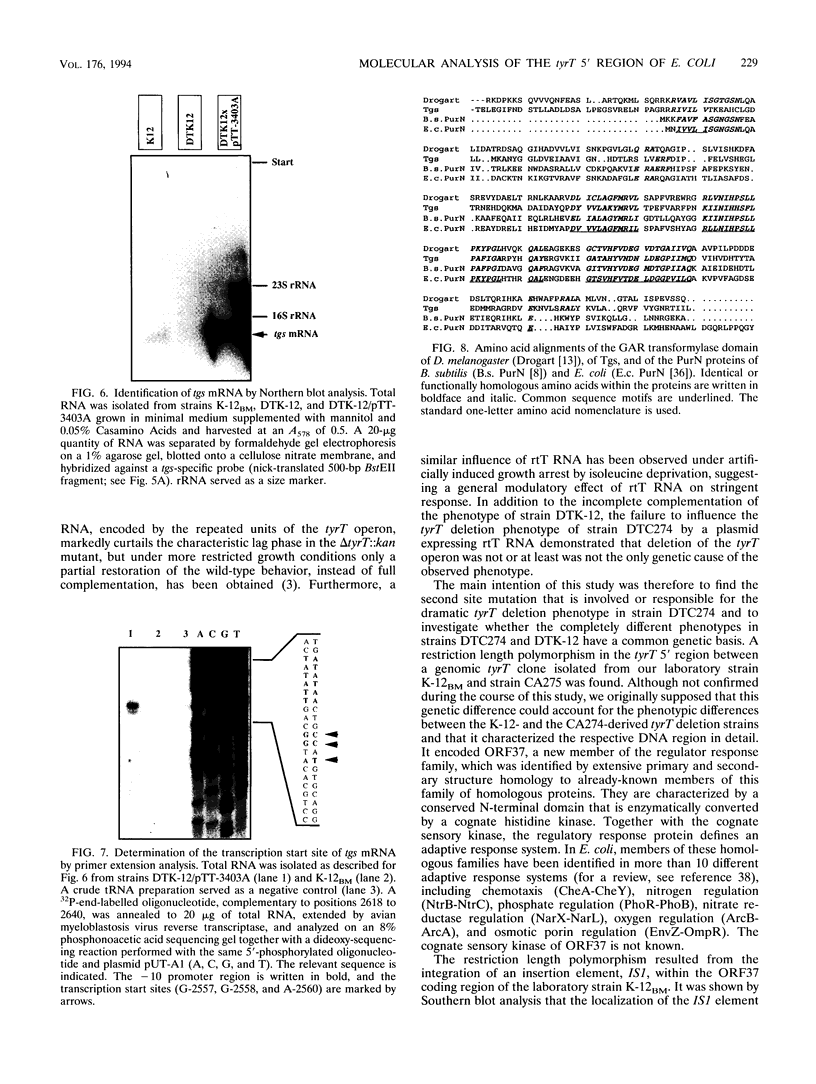
A delta tyrT::kan mutant from Escherichia coli K-12 (DTK-12) shows a transient growth lag that is caused by glycine starvation (U. Michelsen, M. Bösl, T. Dingermann, and H. Kersten, J. Bacteriol. 171:5987-5994, 1989). The same deletion, transduced into the relA1 spoT1 mutant CA274 to construct strain DTC274, caused complete growth inhibition in glucose minimal medium. Here, we show that the tyrT 5' region contains three new open reading frames in the order ORF37-->ORF34-->ORF32-->tyrT and that the delta tyrT::kan allele used previously deletes tyrT as well as a carboxy-terminal portion of ORF32. A plasmid encoding ORF32 totally complemented the inability of strain DTC274 to grow on glucose minimal medium as well as the transient glycine starvation phenomenon in DTK-12, and ORF32 was designated tgs. Partial deletion of tgs, cotransduced with the marker delta tyrT::kan, was responsible for the completely different phenotypes of the deletion mutants DTK-12 and DTC274. The deduced Tgs protein sequence showed significant homology to the PurN protein of E. coli and to enzymes with glycinamide ribonucleotide transformylase activity. We discuss whether growth inhibition in strain DTC274 may be caused by synergistic effects with the preexisting mutations relA1 and spoT1. The deduced protein sequence of ORF37 showed striking similarity to regulator response proteins and is probably a new member of this family. A spontaneous mutation in ORF37, caused by the integration of an insertion element, IS1, exhibited no phenotype.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimi J., Qiu H., Williams J., Zalkin H., Dixon J. E. De novo purine nucleotide biosynthesis: cloning of human and avian cDNAs encoding the trifunctional glycinamide ribonucleotide synthetase-aminoimidazole ribonucleotide synthetase-glycinamide ribonucleotide transformylase by functional complementation in E. coli. Nucleic Acids Res. 1990 Nov 25;18(22):6665–6672. doi: 10.1093/nar/18.22.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl R. P., Vielmetter W. Complete maps of IS1, IS2, IS3, IS4, IS5, IS30 and IS150 locations in Escherichia coli K12. Mol Gen Genet. 1989 Dec;220(1):147–153. doi: 10.1007/BF00260869. [DOI] [PubMed] [Google Scholar]
- Brendel V., Trifonov E. N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984 May 25;12(10):4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösl M., Kersten H. A novel RNA product of the tyrT operon of Escherichia coli. Nucleic Acids Res. 1991 Nov 11;19(21):5863–5870. doi: 10.1093/nar/19.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbole D. J., Zalkin H. Cloning and characterization of a 12-gene cluster from Bacillus subtilis encoding nine enzymes for de novo purine nucleotide synthesis. J Biol Chem. 1987 Jun 15;262(17):8274–8287. [PubMed] [Google Scholar]
- Egan J., Landy A. Structural analysis of the tRNA1Tyr gene of Escherichia coli. A 178 base pair sequence that is repeated 3.14 times. J Biol Chem. 1978 May 25;253(10):3607–3622. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Eghtedarzadeh M. K. Conserved arrangement of nested genes at the Drosophila Gart locus. Genetics. 1987 Dec;117(4):711–725. doi: 10.1093/genetics/117.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Keene M. A., Sloan J. S., Bleskan J., Hards R., Patterson D. Multiple purine pathway enzyme activities are encoded at a single genetic locus in Drosophila. Proc Natl Acad Sci U S A. 1986 Feb;83(3):720–724. doi: 10.1073/pnas.83.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hernandez V. J., Bremer H. Escherichia coli ppGpp synthetase II activity requires spoT. J Biol Chem. 1991 Mar 25;266(9):5991–5999. [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Genetically separable functional elements mediate the optimal expression and stringent regulation of a bacterial tRNA gene. Cell. 1985 Feb;40(2):319–326. doi: 10.1016/0092-8674(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Requirement for an upstream element for optimal transcription of a bacterial tRNA gene. Nature. 1983 Sep 15;305(5931):248–250. doi: 10.1038/305248a0. [DOI] [PubMed] [Google Scholar]
- Lejeune P., Bertin P., Walon C., Willemot K., Colson C., Danchin A. A locus involved in kanamycin, chloramphenicol and L-serine resistance is located in the bglY-galU region of the Escherichia coli K12 chromosome. Mol Gen Genet. 1989 Aug;218(2):361–363. doi: 10.1007/BF00331292. [DOI] [PubMed] [Google Scholar]
- Leonardo M. R., Clark D. P. Locations of genes in the nar-adhE region of the Escherichia coli K-12 chromosome. J Bacteriol. 1991 Mar;173(5):1574–1575. doi: 10.1128/jb.173.5.1574-1575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkle G. M., Altman S. Large deletion mutants of Escherichia coli tRNATyr1. J Mol Biol. 1982 Feb 25;155(2):83–103. doi: 10.1016/0022-2836(82)90438-7. [DOI] [PubMed] [Google Scholar]
- Metzger S., Schreiber G., Aizenman E., Cashel M., Glaser G. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J Biol Chem. 1989 Dec 15;264(35):21146–21152. [PubMed] [Google Scholar]
- Michelsen U., Bösl M., Dingermann T., Kersten H. The tyrT locus of Escherichia coli exhibits a regulatory function for glycine metabolism. J Bacteriol. 1989 Nov;171(11):5987–5994. doi: 10.1128/jb.171.11.5987-5994.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. T., Hill K. A., Schimmel P. Evidence for a "cysteine-histidine box" metal-binding site in an Escherichia coli aminoacyl-tRNA synthetase. Biochemistry. 1991 Jul 16;30(28):6970–6976. doi: 10.1021/bi00242a023. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Vanet A., Vijgenboom E., Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990 Mar;9(3):727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard P., Smith J. M. Evidence for a novel glycinamide ribonucleotide transformylase in Escherichia coli. J Bacteriol. 1993 Jun;175(11):3591–3597. doi: 10.1128/jb.175.11.3591-3597.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Nucleotide sequence of an insertion element, IS1. Proc Natl Acad Sci U S A. 1978 Feb;75(2):615–619. doi: 10.1073/pnas.75.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Autoregulation of Escherichia coli purR requires two control sites downstream of the promoter. J Bacteriol. 1990 Oct;172(10):5758–5766. doi: 10.1128/jb.172.10.5758-5766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. J., Landy A. Structure and organization of the two tRNATyr gene clusters on the E. coli chromosome. Cell. 1979 Mar;16(3):523–534. doi: 10.1016/0092-8674(79)90027-8. [DOI] [PubMed] [Google Scholar]
- Rossi J., Egan J., Hudson L., Landy A. The tyrT locus: termination and processing of a complex transcript. Cell. 1981 Nov;26(3 Pt 1):305–314. doi: 10.1016/0092-8674(81)90199-9. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S. E., Burke J. F. Rapid isolation of miniprep DNA for double strand sequencing. Nucleic Acids Res. 1990 Aug 25;18(16):4948–4948. doi: 10.1093/nar/18.16.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A., Higgins N. P. Differential activity of a transposable element in Escherichia coli colonies. J Bacteriol. 1989 Nov;171(11):5975–5986. doi: 10.1128/jb.171.11.5975-5986.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Daum H. A., 3rd Identification and nucleotide sequence of a gene encoding 5'-phosphoribosylglycinamide transformylase in Escherichia coli K12. J Biol Chem. 1987 Aug 5;262(22):10565–10569. [PubMed] [Google Scholar]
- Steiert J. G., Rolfes R. J., Zalkin H., Stauffer G. V. Regulation of the Escherichia coli glyA gene by the purR gene product. J Bacteriol. 1990 Jul;172(7):3799–3803. doi: 10.1128/jb.172.7.3799-3803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Mace H. A., Berman M. L. RNA polymerase interactions with the upstream region of the E. coli tyrT promoter. Cell. 1983 Nov;35(1):265–273. doi: 10.1016/0092-8674(83)90229-5. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Weeks J. R. Alteration of the growth-rate-dependent regulation of Escherichia coli tyrT expression by promoter mutations. J Mol Biol. 1986 May 5;189(1):251–255. doi: 10.1016/0022-2836(86)90397-9. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Mapping of insertion elements IS1, IS2 and IS3 on the Escherichia coli K-12 chromosome. Role of the insertion elements in formation of Hfrs and F' factors and in rearrangement of bacterial chromosomes. J Mol Biol. 1989 Aug 20;208(4):601–614. doi: 10.1016/0022-2836(89)90151-4. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., Turnbough C. L., Jr Role of the purine repressor in the regulation of pyrimidine gene expression in Escherichia coli K-12. J Bacteriol. 1990 Jun;172(6):3208–3213. doi: 10.1128/jb.172.6.3208-3213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Kalman M., Ikehara K., Zemel S., Glaser G., Cashel M. Residual guanosine 3',5'-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem. 1991 Mar 25;266(9):5980–5990. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]