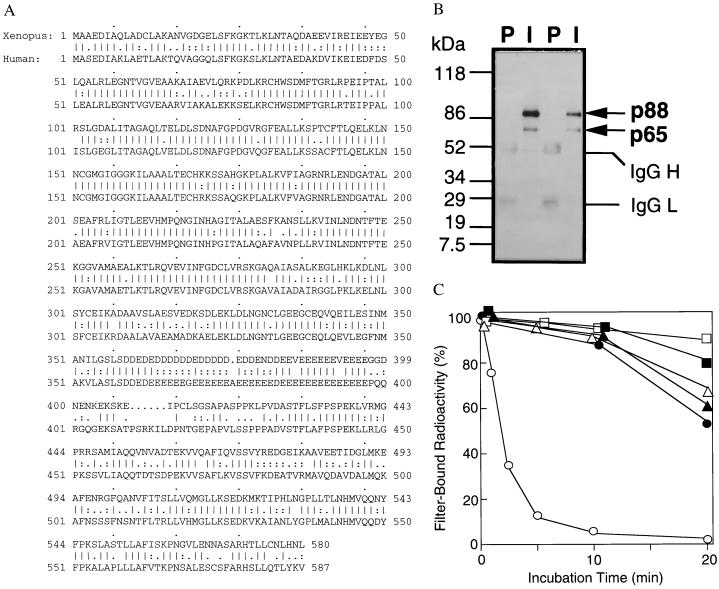
Figure 2.
p88 is a modified form of RanGAP1. (A) Protein sequence of Xenopus RanGAP1. The protein sequence of human RanGAP1 is shown below for comparison. Straight vertical lines indicate identical amino acids. Double dots indicate conserved residues, and single dots indicate charged residues. Gaps inserted for sequence alignment are indicated by periods. (B) p88 is a modified form of RanGAP1. Immunoprecipitations were performed from egg extracts using affinity-purified anti-RanGAP1 antibodies (I) or preimmune sera (P) from two rabbits. The precipitated proteins were analyzed by Western blotting with affinity-purified anti-RanGAP1 antibodies from one rabbit. The migration of molecular mass standards (in kDa) is indicated on the left. The two RanGAP1 bands (p88 and p65) and the IgG H and L chains are indicated on the right. (C) p340RanBP2–p88–p18 complexes contain RanGAP activity. Beads (1 μl) from an anti-p340RanBP2 immunoprecipitation (○ and •), a preimmune control incubation (▵ and ▴), or an incubation with protein A-Sepharose lacking IgG (□ and ▪) were added to GAP buffer containing 100 nM Ran–[γ-32P]GTP (○, ▵, and □) or 100 nM Ran–[α-32P]GTP (•, ▴, and ▪). At the indicated times, protein-associated 32P was measured in a filter binding assay.