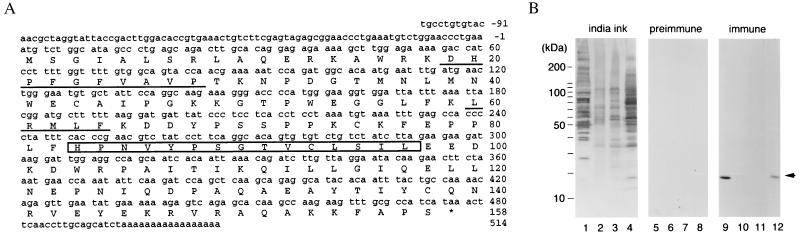
Figure 3.
Cloning of Xenopus UBC9 and preparation of anti-Ubc9p antibodies. (A) Nucleotide and predicted amino acid sequence of Xenopus laevis UBC9 cDNA. The amino acid sequences obtained by peptide sequencing of p18 are underlined. The E2 active site motif, HPN(I/V)X3–4GX(I/V/L)C(I/L)X(I/V)(I/L) is boxed. (B) Xenopus egg cytosolic fraction (1 μl; lanes 1, 5, and 9), 0.1 μl of membrane faction (lanes 2, 6, and 10), or 0.1 μl of glycogen pellet fraction (lanes 3, 7, and 11) were blotted to poly(vinylidene difluoride) membranes for analysis. On the same membranes, 30 μg of total protein from Xenopus A6 tissue culture cells was also blotted (lanes 4, 8, and 12). One membrane was stained with India ink to show the transfer of proteins to the membrane (lanes 1–4). The other filters were subjected to Western blot analysis using preimmune sera (lanes 5–8) or rabbit polyclonal antiserum prepared against recombinant Xenopus Ubc9p (lanes 9–12) at a dilution of 1:5,000. The migration of molecular mass standards (in kDa) are indicated to the left in A and B. Egg interphase cytosol, membrane, and glycogen-rich fractions were prepared as described in Smythe and Newport (22). The arrow at the right indicates the position of p18.