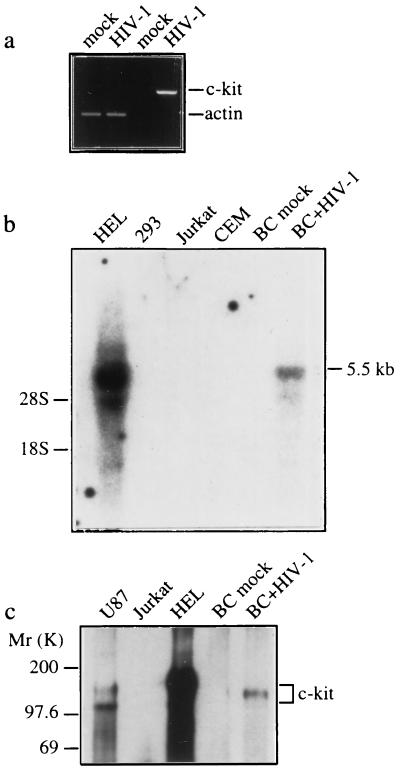
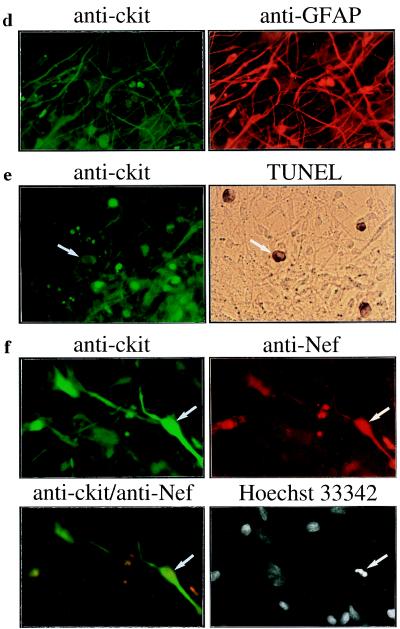
Figure 1.
Induction of c-kit by HIV-1 infection is associated with astrocyte apoptosis. Primary human fetal brain cultures (BC) were infected with HIV-189.6 or mock-infected, and c-Kit expression was examined by RT–PCR (a), Northern blot (b), and Western blot (c) analyses and immunofluorescence staining (d–f) at 30 days after infection. Endogenous c-Kit is detected in HEL and U87 cells but not in Jurkat, CEM, and 293 cells (b and c). (a) RT–PCR analysis of c-kit and β-actin transcripts. (b) Northern blot analysis of c-kit mRNA (5.5 kb) in total RNA (30 μg) isolated from mock- and HIV-1-infected brain cultures. The positions of 18S and 28S ribosomal RNA are indicated. (c) Western blot analysis of c-Kit (145 kDa). The two forms of c-Kit detected in U87 cells most likely correspond to the 120-kDa precursor and 145-kDa mature forms (25). (d–f) Double immunofluorescence staining of HIV-1-infected cultures with the primary antibodies indicated followed by fluorescein isothiocyanate- or rhodamine-secondary antibodies. (d) c-Kit colocalizes with the astrocyte-specific marker anti-glial fibrillary acidic protein. (e) Apoptosis in c-Kit-positive cells detected by TUNEL staining (arrows). (f) Apoptotic nuclear morphology in c-Kit-positive Nef-positive astrocyte (arrows) detected by nuclear staining with Hoechst 33342. (Left) Lower row is a double exposure. Cultures shown in f were treated with recombinant tumor necrosis factor α (10 ng/ml) for 48 h, which was necessary to permit detection of Nef in astrocytes (26). Treatment with tumor necrosis factor α did not change the expression of c-Kit or the relative number of apoptotic astrocytes in mock- versus HIV-1-infected cultures. Original magnifications: ×200 (d), ×300 (e), and ×400 (f). Results in a–f were similar in two or three experiments.