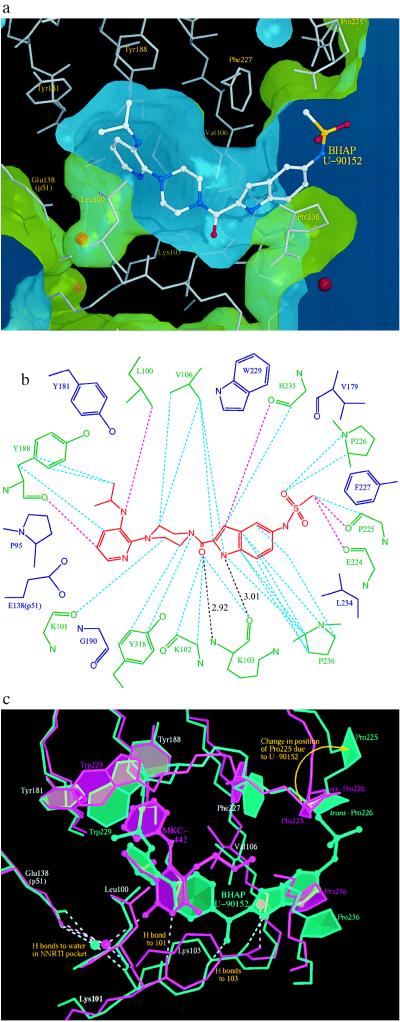
Figure 3.
Positioning of U-90152 in the NNI-binding pocket. (a) The surface of the NNI-binding pocket in the complex with U-90152. The inhibitor is shown as an atom-colored ball-and-stick model with the surrounding protein structure as thin grey sticks. Water molecules are indicated by red spheres. The green face of the surface points toward the protein; the blue face toward the solvent. This is the first RT–NNI complex structure to show the channel connecting the pocket to the solvent and may indicate the mode of entry into the (normally buried) pocket for all NNIs. (b) A diagram of the contacts between U-90152 (in red) and the surrounding protein structure. Specific interactions are shown by broken lines (pink, <3.3 Å; blue, 3.3–3.6 Å; black, hydrogen bonds with distances). Residues contacting U-90152 are shown in green, those observed in contact with NNIs in other structures are in blue. (c) A comparison between the RT complexes with the NNIs U-90152 (green) and MKC-442 (17) (pink). The inhibitor is shown as a ball-and-stick representation, the surrounding protein structure as sticks, water molecules as spheres, and hydrogen bonds as broken lines. To compensate for the (slight) difference in crystal forms the models were superposed based on the surrounding protein structure in the manner we have described (13). Only the pyridine end of U-90152 occupies equivalent volume to MKC-442 (and indeed all other NNIs studied to date). The novel hydrogen bonding pattern and significant effects on Val-106, Pro-225, and Pro-236 are clearly shown.