Abstract
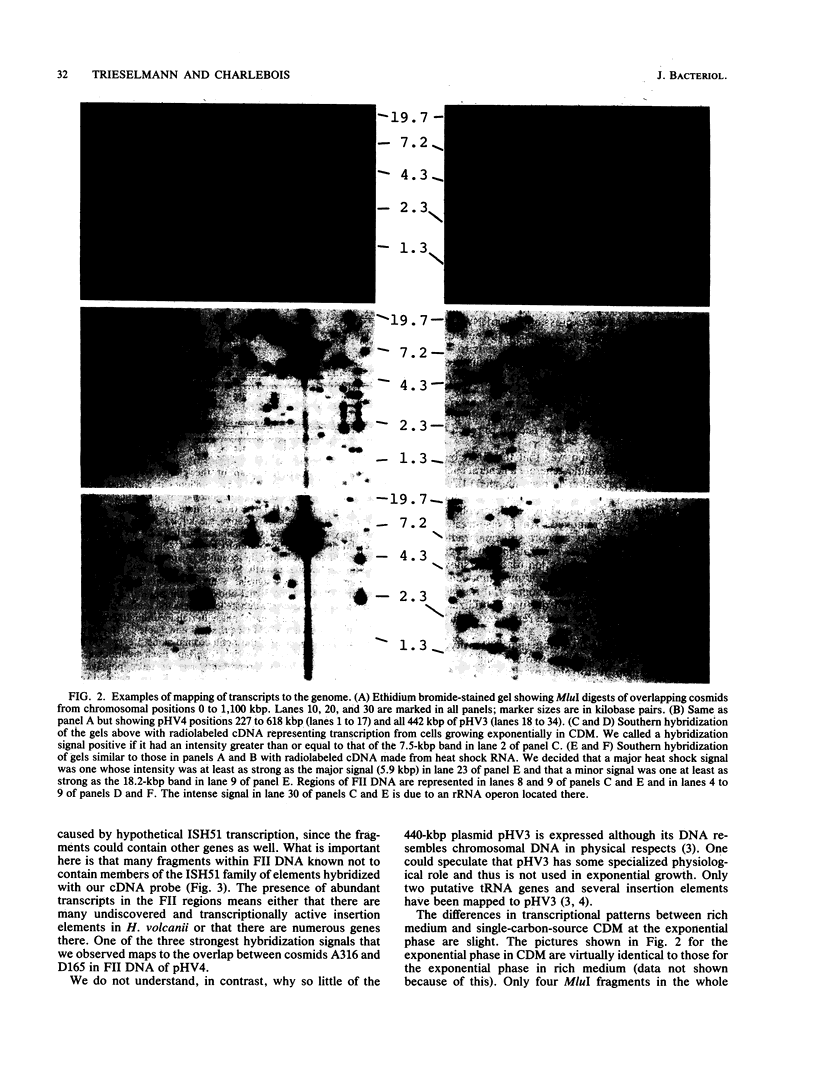
Transcriptionally active regions of the Haloferax volcanii genome were mapped by hybridization of radiolabeled cDNA to Southern blots of our minimal set of overlapping cosmid clones covering 96% of the 4.1-Mbp genome. Transcription during exponential growth occurred in nearly every region of the 2,920-kbp chromosome. Large parts of the 690- and the 86-kbp plasmids were transcribed, but the 440-kbp plasmid showed little expression. Transcription after a 40-min heat shock at 65 degrees C was generally reduced, apart from a small set of strongly expressed loci all situated on the chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. Rapid extraction of high molecular weight RNA from cultured cells and granulocytes for Northern analysis. Nucleic Acids Res. 1988 Feb 25;16(4):1487–1497. doi: 10.1093/nar/16.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover R. K., Doolittle W. F. Characterization of a gene involved in histidine biosynthesis in Halobacterium (Haloferax) volcanii: isolation and rapid mapping by transformation of an auxotroph with cosmid DNA. J Bacteriol. 1990 Jun;172(6):3244–3249. doi: 10.1128/jb.172.6.3244-3249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., McKee A. H., Doolittle W. F. Archaebacterial heat-shock proteins. EMBO J. 1984 Apr;3(4):745–749. doi: 10.1002/j.1460-2075.1984.tb01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman J. D., Schalkwyk L. C., Doolittle W. F. ISH51: a large, degenerate family of insertion sequence-like elements in the genome of the archaebacterium, Halobacterium volcanii. Nucleic Acids Res. 1986 Sep 11;14(17):6983–7000. doi: 10.1093/nar/14.17.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W. L., Cohen A., Tsouluhas D., Doolittle W. F. Genes for tryptophan biosynthesis in the archaebacterium Haloferax volcanii. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6614–6618. doi: 10.1073/pnas.87.17.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., McCarthy B. J. Characterization of the deoxyribonucleic acid of various strains of halophilic bacteria. J Bacteriol. 1969 Jul;99(1):248–254. doi: 10.1128/jb.99.1.248-254.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza C., Doolittle W. F. Unusual physical organization of the Halobacterium genome. Nature. 1982 Feb 4;295(5848):384–389. doi: 10.1038/295384a0. [DOI] [PubMed] [Google Scholar]



