Abstract
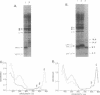
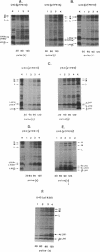
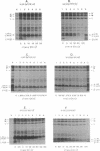
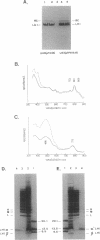
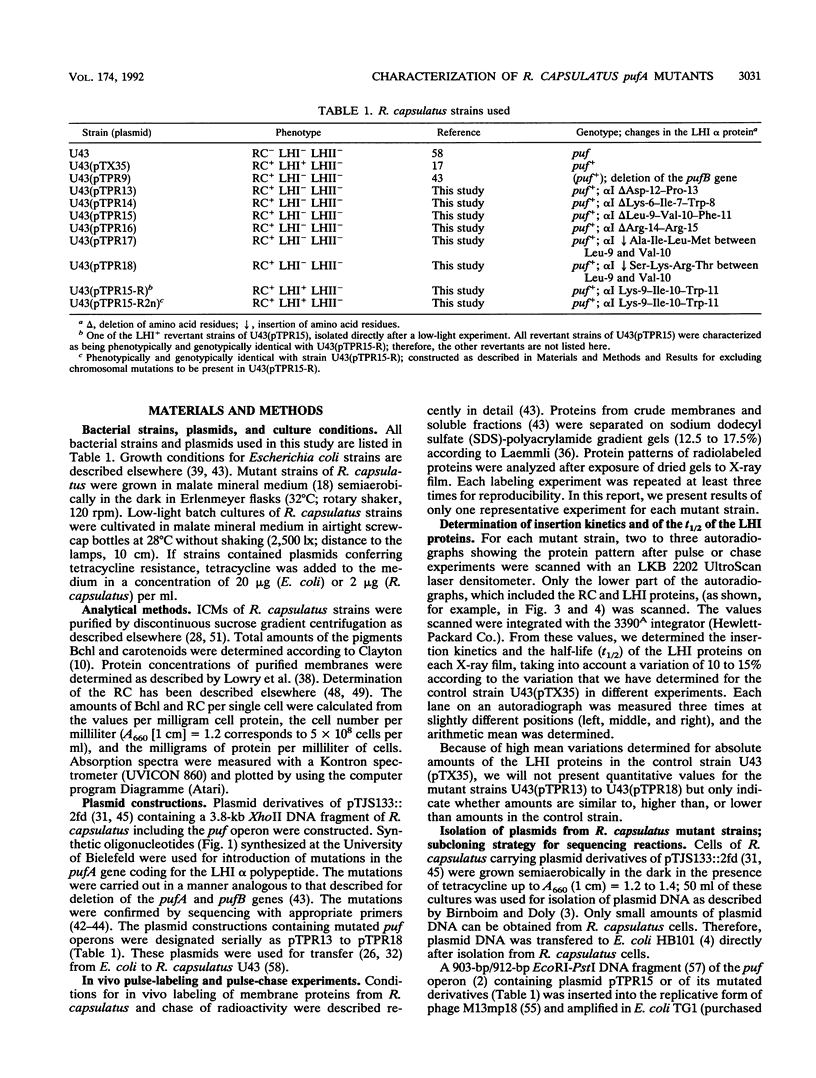
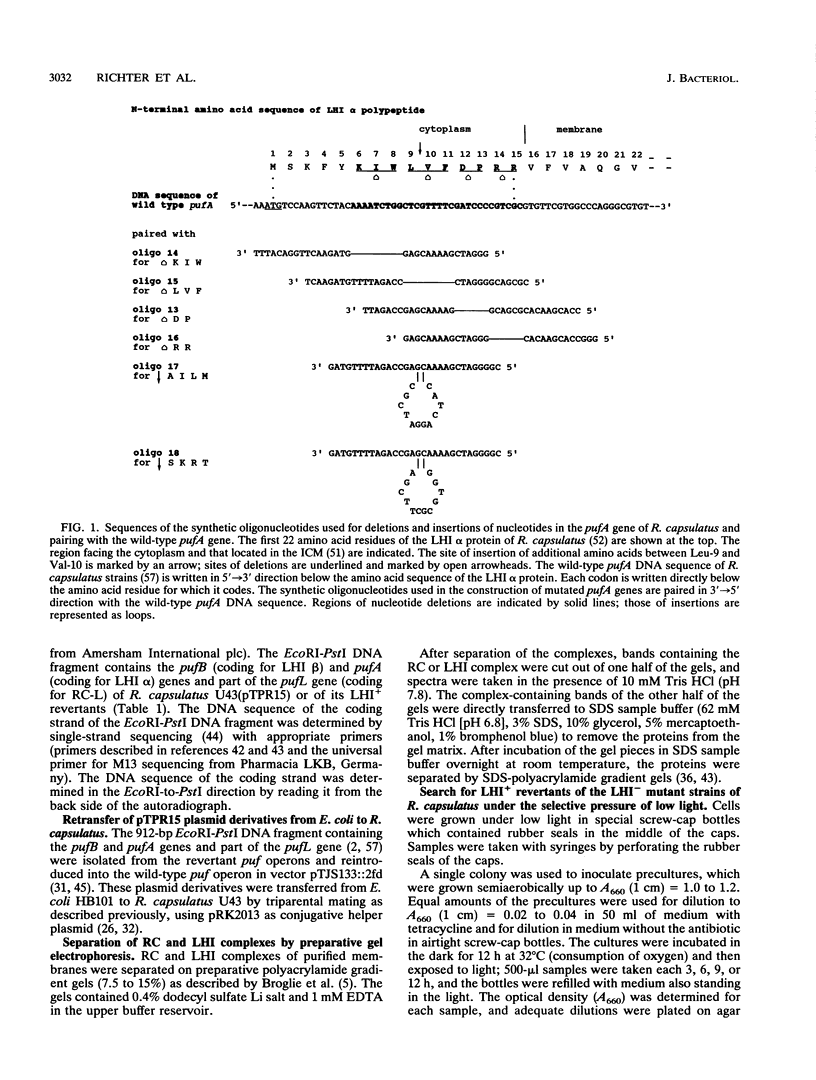
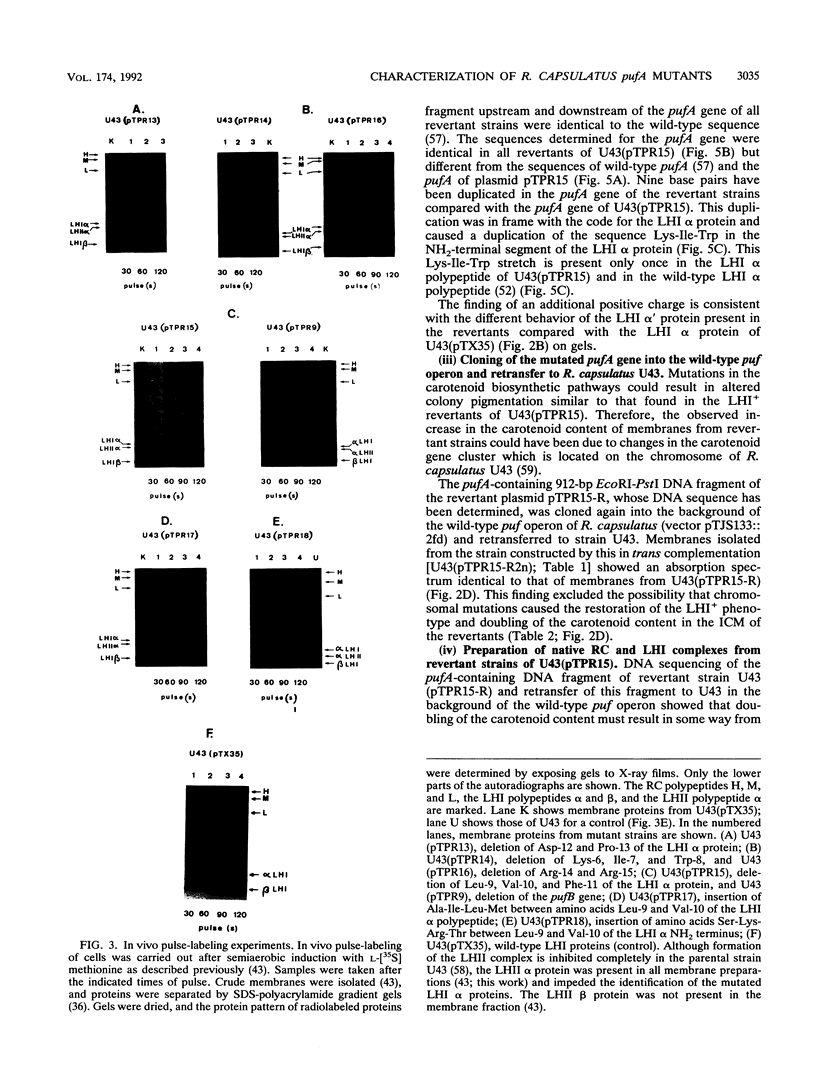
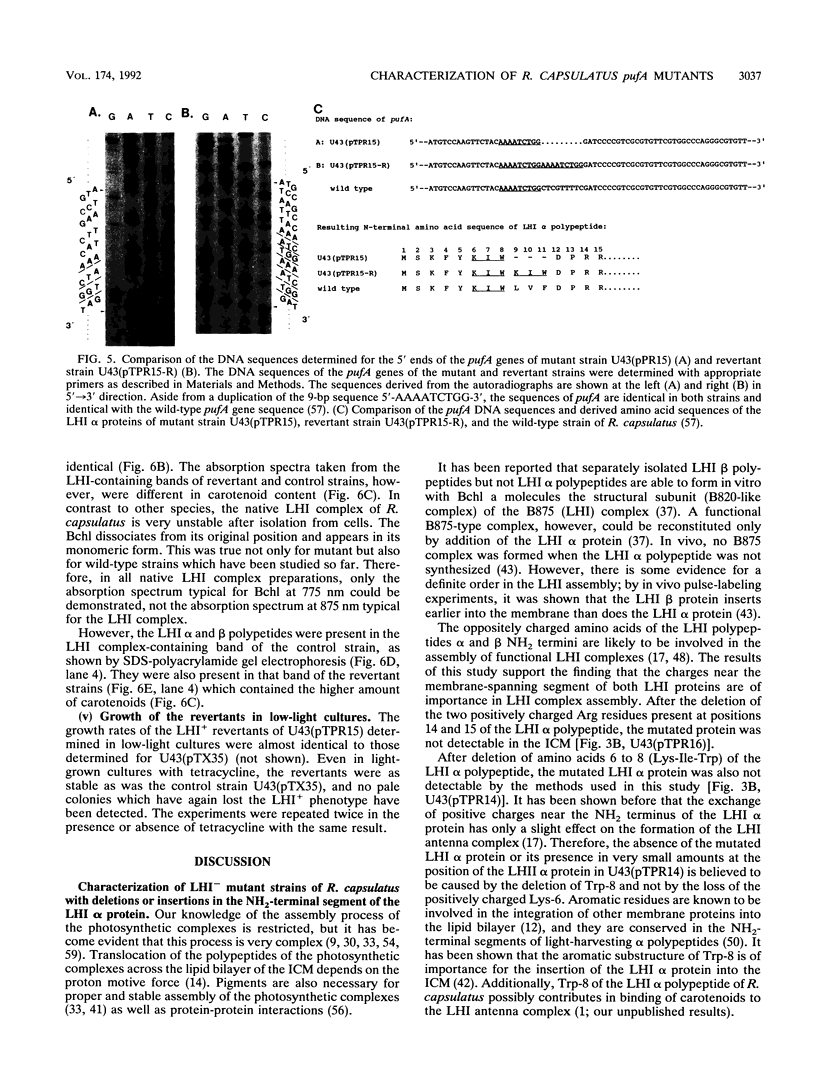
The NH2 termini of light-harvesting complex I (LHI) polypeptides alpha and beta of Rhodobacter capsulatus are thought to be involved in the assembly of the LHI complex. For a more detailed study of the role of the NH2-terminal segment of the LHI alpha protein in insertion into the intracytoplasmic membrane (ICM) of R. capsulatus, amino acids 6 to 8, 9 to 11, 12 and 13, or 14 and 15 of the LHI alpha protein were deleted. Additionally, the hydrophobic stretch of the amino acids 7 to 11 was lengthened by insertion of hydrophobic or hydrophilic amino acids. All mutations abolished the ability of the mutant strains to form a functional LHI antenna complex. All changes introduced into the LHI alpha protein strongly reduced the stability of its LHI beta partner protein in the ICM. The effects on the mutated protein itself, however, were different. Deletion of amino acids 6 to 8, 9 to 11, or 14 and 15 drastically reduced the amount of the LHI alpha protein inserted into the membrane or prevented its insertion. Deletion of amino acids 12 and 13 and lengthening of the stretch of amino acids 7 to 11 reduced the half-life of the mutated LHI alpha protein in the ICM in comparison with the wild-type LHI alpha protein. Under the selective pressure of low light, revertants which regained a functional LHI antenna complex were identified only for the mutant strain deleted of amino acids 9 to 11 of the LHI alpha polypeptide [U43 (pTPR15)]. The restoration of the LHI+ phenotype was due to an in-frame duplication of 9 bp in the pufA gene directly upstream of the site of deletion present in strain U43(pTPR15). The duplicated nucleotides code for the amino acids Lys, Ile, and Trp. Membranes purified from the revertants were different from that of the reaction center-positive LHI+ LHII- control strain U43(pTX35) in doubling of the carotenoid content and increase of the size of the photosynthetic unit. By separating the reaction center and LHI complexes of the revertants by native preparative gel electrophoresis, we confirmed that the higher amount of carotenoids was associated with the LHI proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babst M., Albrecht H., Wegmann I., Brunisholz R., Zuber H. Single amino acid substitutions in the B870 alpha and beta light-harvesting polypeptides of Rhodobacter capsulatus. Structural and spectral effects. Eur J Biochem. 1991 Dec 5;202(2):277–284. doi: 10.1111/j.1432-1033.1991.tb16373.x. [DOI] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Broglie R. M., Hunter C. N., Delepelaire P., Niederman R. A., Chua N. H., Clayton R. K. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Donohue T. J., Varga A. R., Staehelin L. A., Kaplan S. Induction of the photosynthetic membranes of Rhodopseudomonas sphaeroides: biochemical and morphological studies. J Bacteriol. 1984 Aug;159(2):540–554. doi: 10.1128/jb.159.2.540-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell R. J., Frank H. A. How carotenoids function in photosynthetic bacteria. Biochim Biophys Acta. 1987;895(2):63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- Davidson E., Cogdell R. J. Reconstitution of carotenoids into the light-harvesting pigment-protein complex from the carotenoidless mutant of Rhodopseudomonas as sphaeroides R26. Biochim Biophys Acta. 1981 Apr 13;635(2):295–303. doi: 10.1016/0005-2728(81)90028-1. [DOI] [PubMed] [Google Scholar]
- Dierstein R., Drews G. Effect of uncoupler on assembly pathway for pigment-binding protein of bacterial photosynthetic membranes. J Bacteriol. 1986 Oct;168(1):167–172. doi: 10.1128/jb.168.1.167-172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Hoger J. H., Kaplan S. Cloning and expression of the Rhodobacter sphaeroides reaction center H gene. J Bacteriol. 1986 Nov;168(2):953–961. doi: 10.1128/jb.168.2.953-961.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G. Structure and functional organization of light-harvesting complexes and photochemical reaction centers in membranes of phototrophic bacteria. Microbiol Rev. 1985 Mar;49(1):59–70. doi: 10.1128/mr.49.1.59-70.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörge B., Klug G., Gad'on N., Cohen S. N., Drews G. Effects on the formation of antenna complex B870 of Rhodobacter capsulatus by exchange of charged amino acids in the N-terminal domain of the alpha and beta pigment-binding proteins. Biochemistry. 1990 Aug 21;29(33):7754–7758. doi: 10.1021/bi00485a026. [DOI] [PubMed] [Google Scholar]
- Farmilo A., Wilkinson F. On the mechanism of quenching of singlet oxygen in solution. Photochem Photobiol. 1973 Dec;18(6):447–450. doi: 10.1111/j.1751-1097.1973.tb06448.x. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G. A DNA sequence upstream of the puf operon of Rhodobacter capsulatus is involved in its oxygen-dependent regulation and functions as a protein binding site. Mol Gen Genet. 1991 Apr;226(1-2):167–176. doi: 10.1007/BF00273600. [DOI] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Pleiotropic effects of localized Rhodobacter capsulatus puf operon deletions on production of light-absorbing pigment-protein complexes. J Bacteriol. 1988 Dec;170(12):5814–5821. doi: 10.1128/jb.170.12.5814-5821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Drews G. Construction of a gene bank of Rhodopseudomonas capsulata using a broad host range DNA cloning system. Arch Microbiol. 1984 Nov;139(4):319–325. doi: 10.1007/BF00408373. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P., Cortez N., Drews G. Possible role of the highly conserved amino acids Trp-8 and Pro-13 in the N-terminal segment of the pigment-binding polypeptide LHI alpha of Rhodobacter capsulatus. FEBS Lett. 1991 Jul 8;285(1):80–84. doi: 10.1016/0014-5793(91)80729-m. [DOI] [PubMed] [Google Scholar]
- Richter P., Drews G. Incorporation of light-harvesting complex I alpha and beta polypeptides into the intracytoplasmic membrane of Rhodobacter capsulatus. J Bacteriol. 1991 Sep;173(17):5336–5345. doi: 10.1128/jb.173.17.5336-5345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser T. J., Helinski D. R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985 Oct;164(1):446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E., Donohue T. J., Varga A. R., Kaplan S. Control of photosynthetic membrane assembly in Rhodobacter sphaeroides mediated by puhA and flanking sequences. J Bacteriol. 1989 Jan;171(1):436–446. doi: 10.1128/jb.171.1.436-446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehle H., Cortez N., Klug G., Drews G. A negatively charged N terminus in the alpha polypeptide inhibits formation of light-harvesting complex I in Rhodobacter capsulatus. J Bacteriol. 1990 Dec;172(12):7131–7137. doi: 10.1128/jb.172.12.7131-7137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Tadros M. H., Suter F., Seydewitz H. H., Witt I., Zuber H., Drews G. Isolation and complete amino-acid sequence of the small polypeptide from light-harvesting pigment-protein complex I (B870) of Rhodopseudomonas capsulata. Eur J Biochem. 1984 Jan 2;138(1):209–212. doi: 10.1111/j.1432-1033.1984.tb07902.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Komiya H., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26: membrane-protein interactions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6438–6442. doi: 10.1073/pnas.84.18.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S., Bylina E. J. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38(1-3):19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Hearst J. E. Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7613–7617. doi: 10.1073/pnas.83.20.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]