Abstract
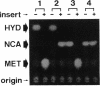
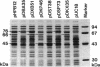
Pseudomonas sp. strain NS671, which produces L-amino acids asymmetrically from the corresponding racemic 5-substituted hydantoins, harbored a plasmid of 172 kb. Curing experiments suggest that this plasmid, designated pHN671, is responsible for the conversion of 5-substituted hydantoins to their corresponding L-amino acids by strain NS671. DNA fragments containing the genes involved in this conversion were cloned from pHN671 in Escherichia coli by using pUC18 as a cloning vector. The smallest recombinant plasmid, designated pHPB12, contained a 7.5-kb insert DNA. The nucleotide sequence of the insert DNA was determined, and three closely spaced open reading frames predicted to encode peptides with molecular masses of 75.6, 64.9, and 45.7 kDa were found. These open reading frames were designated hyuA, hyuB, and hyuC, respectively. Cell extracts from E. coli carrying deletion derivatives of pHPB12 were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the gene products of hyuA, hyuB, and hyuC were identified. The functions of these gene products were also examined with the deletion derivatives. The results indicate that both hyuA and hyuB are involved in the conversions of D- and L-5-substituted hydantoins to corresponding N-carbamyl-D- and N-carbamyl-L-amino acids, respectively, and that hyuC is involved in the conversion of N-carbamyl-L-amino acids to L-amino acids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausubel F. M. Regulation of nitrogen fixation genes. Cell. 1984 May;37(1):5–6. doi: 10.1016/0092-8674(84)90294-0. [DOI] [PubMed] [Google Scholar]
- Bennett L. T., Cannon F., Dean D. R. Nucleotide sequence and mutagenesis of the nifA gene from Azotobacter vinelandii. Mol Microbiol. 1988 May;2(3):315–321. doi: 10.1111/j.1365-2958.1988.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Szeto W. W., Lemley P. V., Orme-Johnson W. H., Ausubel F. M. Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res. 1985 Jun 25;13(12):4539–4555. doi: 10.1093/nar/13.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish E. C., Argyropoulos V. P., Pittard J., Davidson B. E. Structure of the Escherichia coli K12 regulatory gene tyrR. Nucleotide sequence and sites of initiation of transcription and translation. J Biol Chem. 1986 Jan 5;261(1):403–410. [PubMed] [Google Scholar]
- Drummond M., Clements J., Merrick M., Dixon R. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):302–307. doi: 10.1038/301302a0. [DOI] [PubMed] [Google Scholar]
- Grönger P., Manian S. S., Reiländer H., O'Connell M., Priefer U. B., Pühler A. Organization and partial sequence of a DNA region of the Rhizobium leguminosarum symbiotic plasmid pRL6JI containing the genes fixABC, nifA, nifB and a novel open reading frame. Nucleic Acids Res. 1987 Jan 12;15(1):31–49. doi: 10.1093/nar/15.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hirschman J., Wong P. K., Sei K., Keener J., Kustu S. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7525–7529. doi: 10.1073/pnas.82.22.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. P., Magasanik B. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Nucleotide sequence of the regulatory gene xylR of the TOL plasmid from Pseudomonas putida. Gene. 1988 Jun 30;66(2):301–306. doi: 10.1016/0378-1119(88)90366-6. [DOI] [PubMed] [Google Scholar]
- Jiang J., Gu B. H., Albright L. M., Nixon B. T. Conservation between coding and regulatory elements of Rhizobium meliloti and Rhizobium leguminosarum dct genes. J Bacteriol. 1989 Oct;171(10):5244–5253. doi: 10.1128/jb.171.10.5244-5253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Masepohl B., Klipp W., Pühler A. Genetic characterization and sequence analysis of the duplicated nifA/nifB gene region of Rhodobacter capsulatus. Mol Gen Genet. 1988 Apr;212(1):27–37. doi: 10.1007/BF00322441. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miranda-Ríos J., Sánchez-Pescador R., Urdea M., Covarrubias A. A. The complete nucleotide sequence of the glnALG operon of Escherichia coli K12. Nucleic Acids Res. 1987 Mar 25;15(6):2757–2770. doi: 10.1093/nar/15.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees D. W., Stein P. A., Ludwig R. A. The Azorhizobium caulinodans nifA gene: identification of upstream-activating sequences including a new element, the 'anaerobox'. Nucleic Acids Res. 1988 Oct 25;16(20):9839–9853. doi: 10.1093/nar/16.20.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Bergström S., Edlund T., Grundström T., Jaurin B., Lindberg F. P., Olsson O. Overlapping genes. Annu Rev Genet. 1983;17:499–525. doi: 10.1146/annurev.ge.17.120183.002435. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Astwood P. M., Nixon B. T., Ausubel F. M. Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucleic Acids Res. 1987 Oct 12;15(19):7921–7934. doi: 10.1093/nar/15.19.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- STARK G. R., SMYTH D. G. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker K., Reijnders W. N., Oltmann L. F., Stouthamer A. H. Initial cloning and sequencing of hydHG, an operon homologous to ntrBC and regulating the labile hydrogenase activity in Escherichia coli K-12. J Bacteriol. 1989 Aug;171(8):4448–4456. doi: 10.1128/jb.171.8.4448-4456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny B., Fischer H. M., Anthamatten D., Bruderer T., Hennecke H. The symbiotic nitrogen fixation regulatory operon (fixRnifA) of Bradyrhizobium japonicum is expressed aerobically and is subject to a novel, nifA-independent type of activation. Nucleic Acids Res. 1987 Oct 26;15(20):8479–8499. doi: 10.1093/nar/15.20.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegard H. M., Toennies G., Block R. J. Detection of Sulfur-containing Amino Acids on Paper Chromatograms. Science. 1948 Nov 5;108(2810):506–507. doi: 10.1126/science.108.2810.506. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]