Abstract
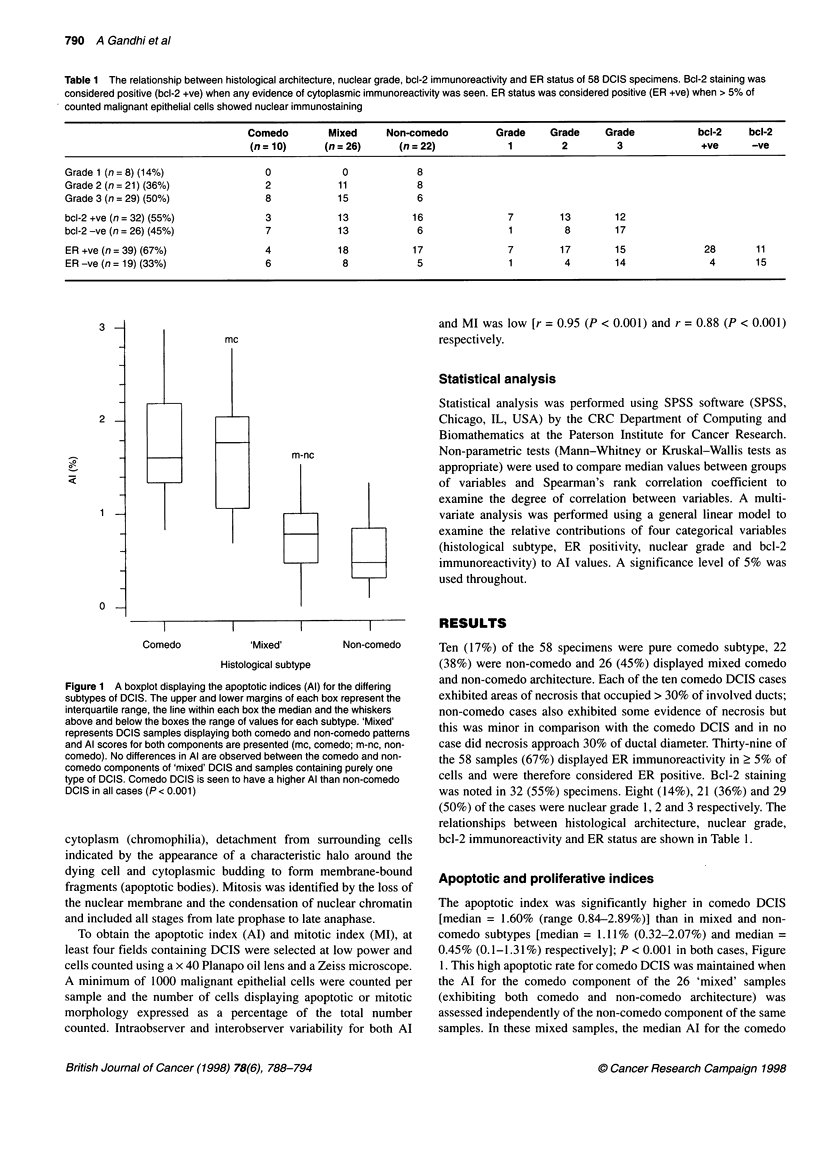
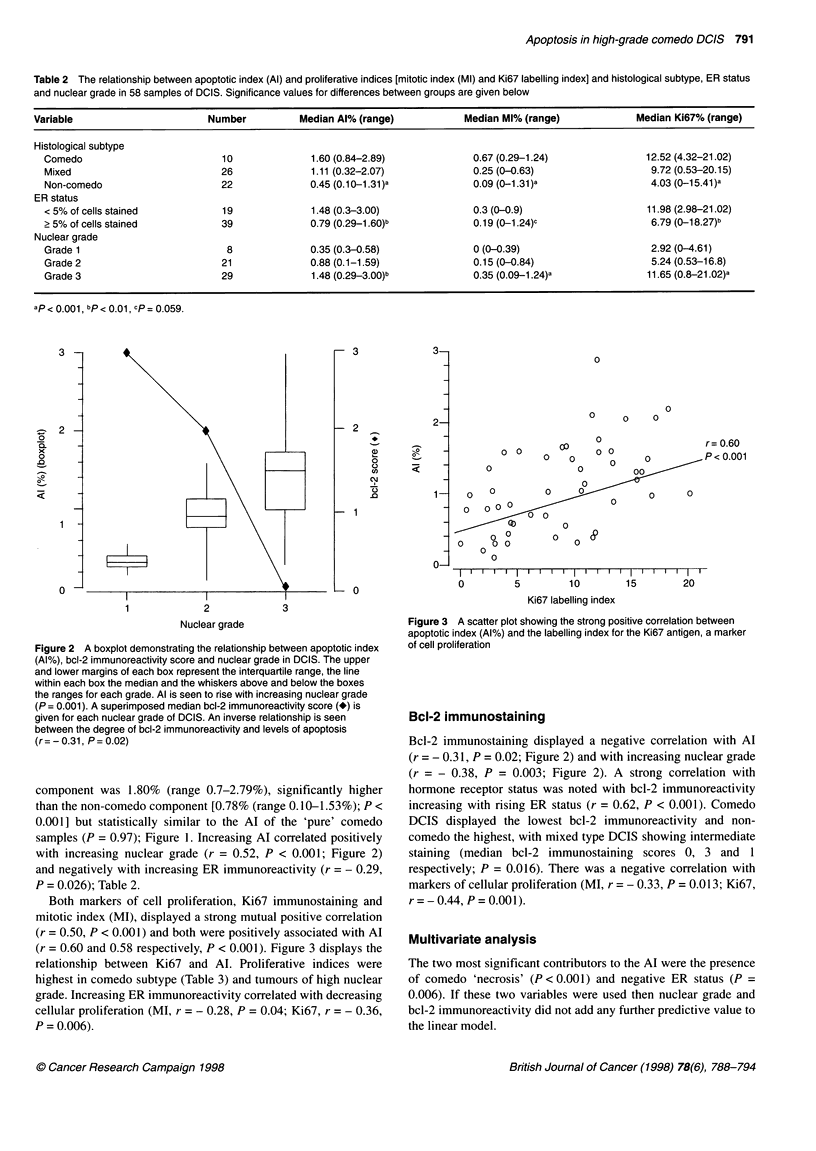
Following breast-conserving surgery for ductal carcinoma in situ (DCIS), the presence of comedo necrosis reportedly predicts for higher rates of post-operative recurrence. To examine the role of programmed cell death (apoptosis) in the aetiology of the cell death described as comedo necrosis, we studied 58 DCIS samples, using light microscopy, for morphological evidence of apoptotic cell death. The percentage of apoptotic cells (apoptotic index, AI) was compared between DCIS with and without evidence of 'comedo necrosis' and related to the immunohistochemical expression of the anti-apoptosis gene bcl-2, mitotic index (MI), the cellular proliferation antigen Ki67, nuclear grade and oestrogen receptor (ER) status. AI was significantly higher in DCIS samples displaying high-grade comedo necrosis than in low-grade non-comedo samples: median AI = 1.60% (range 0.84-2.89%) and 0.45% (0.1-1.31%) respectively (P < 0.001). Increasing nuclear grade correlated positively with AI (P < 0.001) and negatively with bcl-2 expression (P = 0.003). Bcl-2 correlated negatively with AI (P = 0.019) and strongly with ER immunoreactivity (P < 0.001). Cellular proliferation markers (MI and Ki67 immunostaining) correlated strongly with AI and were higher in comedo lesions and tumours of high nuclear grade (P < 0.001 in all cases). Thus, apoptosis contributes significantly to the cell death described in ER-negative, high-grade DCIS in which a high proliferative rate is associated with a high apoptotic rate. It is likely that dysregulation of proliferation/apoptosis control mechanisms accounts for the more malignant features typical of ER negative comedo DCIS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D. J., Howell A., Roberts S. A., Williams G. T., Watson R. J., Coyne J. D., Clarke R. B., Laidlaw I. J., Potten C. S. Reduction in apoptosis relative to mitosis in histologically normal epithelium accompanies fibrocystic change and carcinoma of the premenopausal human breast. J Pathol. 1992 May;167(1):25–32. doi: 10.1002/path.1711670106. [DOI] [PubMed] [Google Scholar]
- Bellamy C. O., McDonald C., Salter D. M., Chetty U., Anderson T. J. Noninvasive ductal carcinoma of the breast: the relevance of histologic categorization. Hum Pathol. 1993 Jan;24(1):16–23. doi: 10.1016/0046-8177(93)90057-n. [DOI] [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Bodis S., Siziopikou K. P., Schnitt S. J., Harris J. R., Fisher D. E. Extensive apoptosis in ductal carcinoma in situ of the breast. Cancer. 1996 May 1;77(9):1831–1835. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1831::AID-CNCR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Deshane J., Grim J., Loechel S., Siegal G. P., Alvarez R. D., Curiel D. T. Intracellular antibody against erbB-2 mediates targeted tumor cell eradication by apoptosis. Cancer Gene Ther. 1996 Mar-Apr;3(2):89–98. [PubMed] [Google Scholar]
- Deshane J., Loechel F., Conry R. M., Siegal G. P., King C. R., Curiel D. T. Intracellular single-chain antibody directed against erbB2 down-regulates cell surface erbB2 and exhibits a selective anti-proliferative effect in erbB2 overexpressing cancer cell lines. Gene Ther. 1994 Sep;1(5):332–337. [PubMed] [Google Scholar]
- Douglas-Jones A. G., Gupta S. K., Attanoos R. L., Morgan J. M., Mansel R. E. A critical appraisal of six modern classifications of ductal carcinoma in situ of the breast (DCIS): correlation with grade of associated invasive carcinoma. Histopathology. 1996 Nov;29(5):397–409. doi: 10.1046/j.1365-2559.1996.d01-513.x. [DOI] [PubMed] [Google Scholar]
- Ernster V. L., Barclay J., Kerlikowske K., Grady D., Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996 Mar 27;275(12):913–918. [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Anderson T. J. Morphological evaluation of cell turnover in relation to the menstrual cycle in the "resting" human breast. Br J Cancer. 1981 Aug;44(2):177–181. doi: 10.1038/bjc.1981.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E. R., Costantino J., Fisher B., Palekar A. S., Redmond C., Mamounas E. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). The National Surgical Adjuvant Breast and Bowel Project Collaborating Investigators. Cancer. 1995 Mar 15;75(6):1310–1319. doi: 10.1002/1097-0142(19950315)75:6<1310::aid-cncr2820750613>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Gee J. M., Robertson J. F., Ellis I. O., Willsher P., McClelland R. A., Hoyle H. B., Kyme S. R., Finlay P., Blamey R. W., Nicholson R. I. Immunocytochemical localization of BCL-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer. 1994 Dec 1;59(5):619–628. doi: 10.1002/ijc.2910590508. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Douglas-Jones A. G., Jasani B., Morgan J. M., Pignatelli M., Mansel R. E. E-cadherin (E-cad) expression in duct carcinoma in situ (DCIS) of the breast. Virchows Arch. 1997 Jan;430(1):23–28. doi: 10.1007/BF01008012. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Hiles I. D., Page M. J., O'Hare M. J. The induction of apoptosis in human mammary luminal epithelial cells by expression of activated c-neu and its abrogation by glucocorticoids. Br J Cancer. 1995 Aug;72(2):386–392. doi: 10.1038/bjc.1995.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Holland P. A., Knox W. F., Potten C. S., Howell A., Anderson E., Baildam A. D., Bundred N. J. Assessment of hormone dependence of comedo ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1997 Jul 16;89(14):1059–1065. doi: 10.1093/jnci/89.14.1059. [DOI] [PubMed] [Google Scholar]
- Holland R., Hendriks J. H., Vebeek A. L., Mravunac M., Schuurmans Stekhoven J. H. Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet. 1990 Mar 3;335(8688):519–522. doi: 10.1016/0140-6736(90)90747-s. [DOI] [PubMed] [Google Scholar]
- Johnston S. R., MacLennan K. A., Sacks N. P., Salter J., Smith I. E., Dowsett M. Modulation of Bcl-2 and Ki-67 expression in oestrogen receptor-positive human breast cancer by tamoxifen. Eur J Cancer. 1994;30A(11):1663–1669. doi: 10.1016/0959-8049(94)00327-2. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lagios M. D. Duct carcinoma in situ. Pathology and treatment. Surg Clin North Am. 1990 Aug;70(4):853–871. doi: 10.1016/s0039-6109(16)45185-6. [DOI] [PubMed] [Google Scholar]
- Leek R. D., Kaklamanis L., Pezzella F., Gatter K. C., Harris A. L. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994 Jan;69(1):135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen P., Aaltomaa S., Kosma V. M., Syrjänen K. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer. 1994;30A(14):2068–2073. doi: 10.1016/0959-8049(94)00342-3. [DOI] [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Watson A. J., Loh D. Y., Nakayama K., Nakayama K., Hickman J. A. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995 Jun;108(Pt 6):2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- Page D. L., Dupont W. D., Rogers L. W., Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982 Feb 15;49(4):751–758. doi: 10.1002/1097-0142(19820215)49:4<751::aid-cncr2820490426>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Page D. L., Lagios M. D. Pathologic analysis of the National Surgical Adjuvant Breast Project (NSABP) B-17 Trial. Unanswered questions remaining unanswered considering current concepts of ductal carcinoma in situ. Cancer. 1995 Mar 15;75(6):1219–1227. doi: 10.1002/1097-0142(19950315)75:6<1219::aid-cncr2820750602>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Potten C. S. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev. 1992 Sep;11(2):179–195. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Watson R. J., Williams G. T., Tickle S., Roberts S. A., Harris M., Howell A. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988 Aug;58(2):163–170. doi: 10.1038/bjc.1988.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S. What is an apoptotic index measuring? A commentary. Br J Cancer. 1996 Dec;74(11):1743–1748. doi: 10.1038/bjc.1996.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman R. A., Cidlowski J. A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993 Apr;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Sierra A., Lloveras B., Castellsagué X., Moreno L., García-Ramirez M., Fabra A. Bcl-2 expression is associated with lymph node metastasis in human ductal breast carcinoma. Int J Cancer. 1995 Jan 3;60(1):54–60. doi: 10.1002/ijc.2910600108. [DOI] [PubMed] [Google Scholar]
- Siziopikou K. P., Prioleau J. E., Harris J. R., Schnitt S. J. bcl-2 expression in the spectrum of preinvasive breast lesions. Cancer. 1996 Feb 1;77(3):499–506. doi: 10.1002/(SICI)1097-0142(19960201)77:3<499::AID-CNCR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Solin L. J., Yeh I. T., Kurtz J., Fourquet A., Recht A., Kuske R., McCormick B., Cross M. A., Schultz D. J., Amalric R. Ductal carcinoma in situ (intraductal carcinoma) of the breast treated with breast-conserving surgery and definitive irradiation. Correlation of pathologic parameters with outcome of treatment. Cancer. 1993 Apr 15;71(8):2532–2542. doi: 10.1002/1097-0142(19930415)71:8<2532::aid-cncr2820710817>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Symonds H., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994 Aug 26;78(4):703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Fukutomi T., Hirohashi S. Pattern of gene alterations in intraductal breast neoplasms associated with histological type and grade. Clin Cancer Res. 1995 Mar;1(3):261–267. [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- van de Vijver M. J., Peterse J. L., Mooi W. J., Wisman P., Lomans J., Dalesio O., Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988 Nov 10;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]


