Abstract
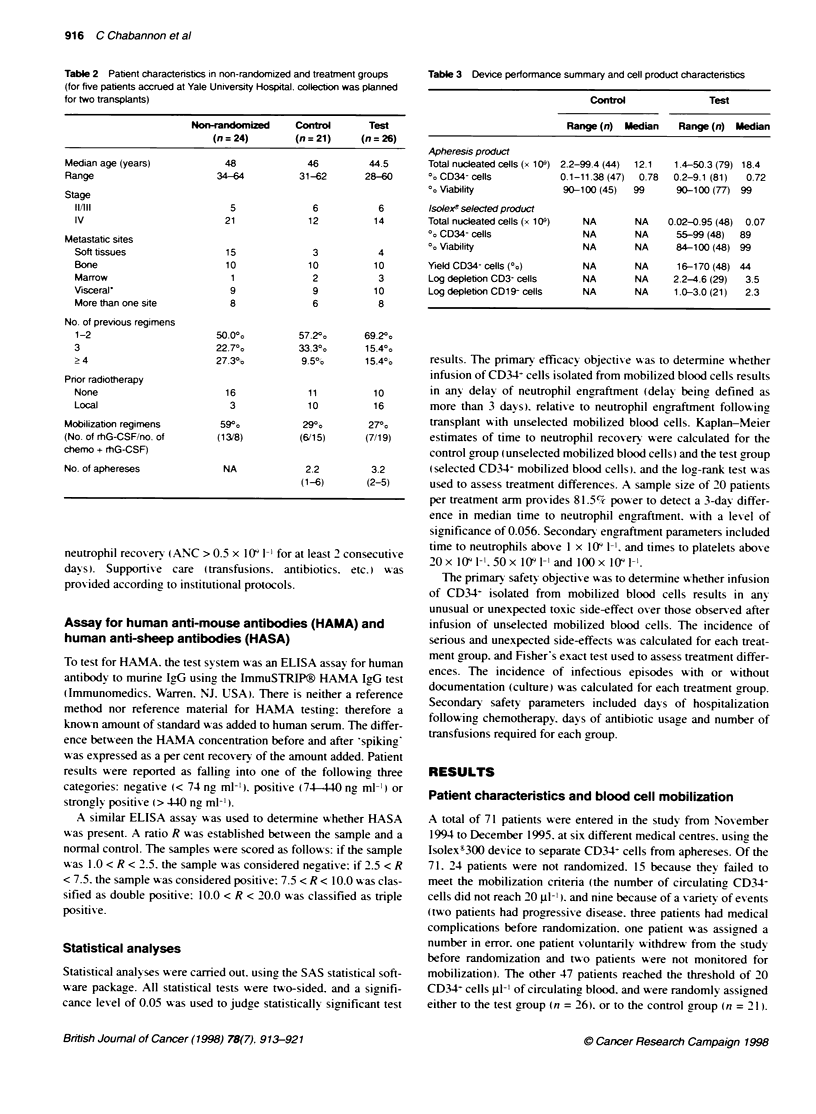
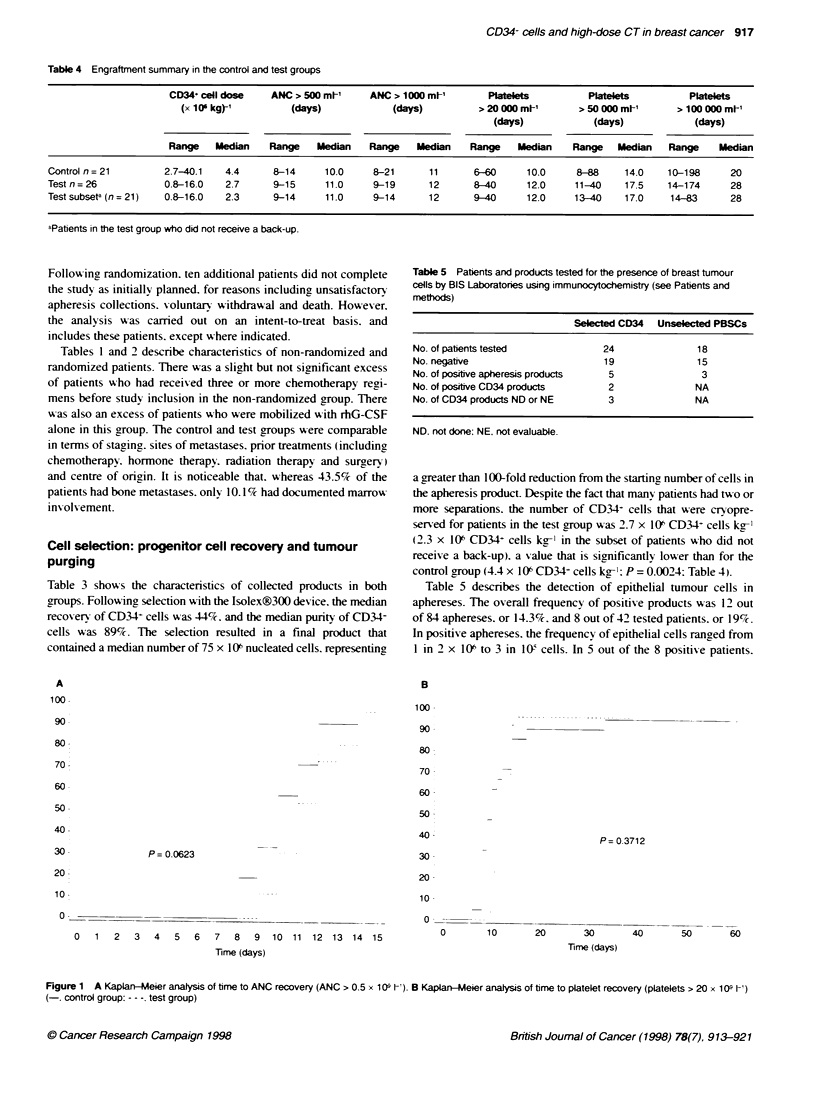
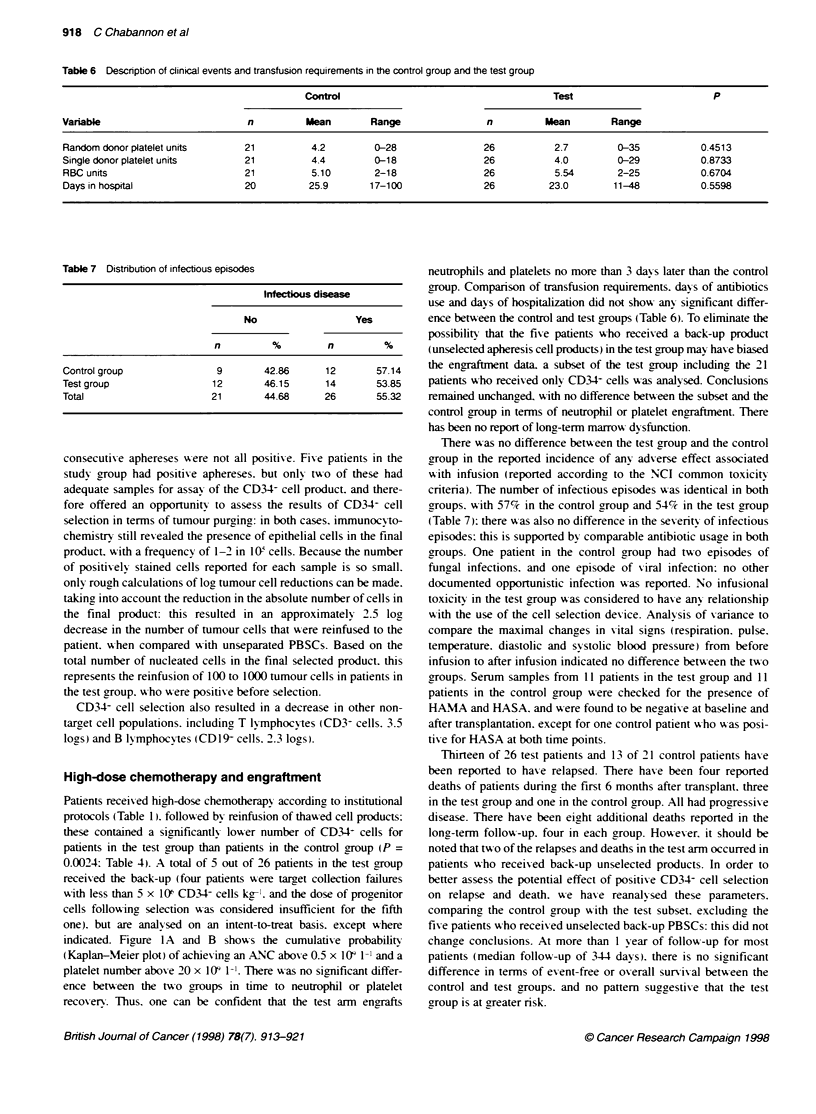
Seventy-one patients with poor-prognosis breast cancer were enrolled after informed consent in a multicentre randomized study to evaluate the use of selected peripheral blood CD34+ cells to support haematopoietic recovery following high-dose chemotherapy. Patients who responded to conventional chemotherapy were mobilized with chemotherapy (mainly high-dose cyclophosphamide) and/or recombinant human granulocyte colony-stimulating factor (rhG-CSF). Patients who reached the threshold of 20 CD34+ cells per microl of peripheral blood underwent apheresis and were randomized at that time to receive either unmanipulated mobilized blood cells or selected CD34+ cells. For patients in the study arm, CD34+ cells were selected from aphereses using the Isolex300 device. Fifteen patients failed to mobilize peripheral blood progenitors and nine other patients were excluded for various reasons. Forty-seven eligible patients were randomized into two comparable groups. CD34+ cells were selected from aphereses in the study group. Haematopoietic recovery occurred at similar times in both groups. No side-effect related to the infusion of selected cells was observed. The frequency of epithelial tumour cells in aphereses was low (8 out of 42 evaluated patients), as determined by immunocytochemistry. We conclude that selected CD34+ cells safely support haematopoietic recovery following high-dose chemotherapy in patients with poor-prognosis breast cancer.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. G., Bryant E. M., Bartelmez S. H., Muirhead D. Y., Knitter G. H., Bensinger W., Strong D. M., Bernstein I. D. CD34+ marrow cells, devoid of T and B lymphocytes, reconstitute stable lymphopoiesis and myelopoiesis in lethally irradiated allogeneic baboons. Blood. 1992 Oct 1;80(7):1693–1701. [PubMed] [Google Scholar]
- Antman K., Ayash L., Elias A., Wheeler C., Hunt M., Eder J. P., Teicher B. A., Critchlow J., Bibbo J., Schnipper L. E. A phase II study of high-dose cyclophosphamide, thiotepa, and carboplatin with autologous marrow support in women with measurable advanced breast cancer responding to standard-dose therapy. J Clin Oncol. 1992 Jan;10(1):102–110. doi: 10.1200/JCO.1992.10.1.102. [DOI] [PubMed] [Google Scholar]
- Baumheter S., Singer M. S., Henzel W., Hemmerich S., Renz M., Rosen S. D., Lasky L. A. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993 Oct 15;262(5132):436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Bender J. G., To L. B., Williams S., Schwartzberg L. S. Defining a therapeutic dose of peripheral blood stem cells. J Hematother. 1992 Winter;1(4):329–341. doi: 10.1089/scd.1.1992.1.329. [DOI] [PubMed] [Google Scholar]
- Bensinger W. I., Buckner C. D., Shannon-Dorcy K., Rowley S., Appelbaum F. R., Benyunes M., Clift R., Martin P., Demirer T., Storb R. Transplantation of allogeneic CD34+ peripheral blood stem cells in patients with advanced hematologic malignancy. Blood. 1996 Dec 1;88(11):4132–4138. [PubMed] [Google Scholar]
- Bensinger W. I., Longin K., Appelbaum F., Rowley S., Weaver C., Lilleby K., Gooley T., Lynch M., Higano T., Klarnet J. Peripheral blood stem cells (PBSCs) collected after recombinant granulocyte colony stimulating factor (rhG-CSF): an analysis of factors correlating with the tempo of engraftment after transplantation. Br J Haematol. 1994 Aug;87(4):825–831. doi: 10.1111/j.1365-2141.1994.tb06744.x. [DOI] [PubMed] [Google Scholar]
- Berenson R. J., Bensinger W. I., Hill R. S., Andrews R. G., Garcia-Lopez J., Kalamasz D. F., Still B. J., Spitzer G., Buckner C. D., Bernstein I. D. Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma. Blood. 1991 Apr 15;77(8):1717–1722. [PubMed] [Google Scholar]
- Bezwoda W. R., Seymour L., Dansey R. D. High-dose chemotherapy with hematopoietic rescue as primary treatment for metastatic breast cancer: a randomized trial. J Clin Oncol. 1995 Oct;13(10):2483–2489. doi: 10.1200/JCO.1995.13.10.2483. [DOI] [PubMed] [Google Scholar]
- Bonadonna G., Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981 Jan 1;304(1):10–15. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- Brenner M. K., Rill D. R., Moen R. C., Krance R. A., Mirro J., Jr, Anderson W. F., Ihle J. N. Gene-marking to trace origin of relapse after autologous bone-marrow transplantation. Lancet. 1993 Jan 9;341(8837):85–86. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- Brugger W., Bross K. J., Glatt M., Weber F., Mertelsmann R., Kanz L. Mobilization of tumor cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumors. Blood. 1994 Feb 1;83(3):636–640. [PubMed] [Google Scholar]
- Brugger W., Henschler R., Heimfeld S., Berenson R. J., Mertelsmann R., Kanz L. Positively selected autologous blood CD34+ cells and unseparated peripheral blood progenitor cells mediate identical hematopoietic engraftment after high-dose VP16, ifosfamide, carboplatin, and epirubicin. Blood. 1994 Sep 1;84(5):1421–1426. [PubMed] [Google Scholar]
- Chabannon C., Le Coroller A. G., Faucher C., Novakovitch G., Blaise D., Moatti J. P., Maraninchi D., Mannoni P. Patient condition affects the collection of peripheral blood progenitors after priming with recombinant granulocyte colony-stimulating factor. J Hematother. 1995 Jun;4(3):171–179. doi: 10.1089/scd.1.1995.4.171. [DOI] [PubMed] [Google Scholar]
- Civin C. I., Trischmann T., Kadan N. S., Davis J., Noga S., Cohen K., Duffy B., Groenewegen I., Wiley J., Law P. Highly purified CD34-positive cells reconstitute hematopoiesis. J Clin Oncol. 1996 Aug;14(8):2224–2233. doi: 10.1200/JCO.1996.14.8.2224. [DOI] [PubMed] [Google Scholar]
- Deisseroth A. B., Zu Z., Claxton D., Hanania E. G., Fu S., Ellerson D., Goldberg L., Thomas M., Janicek K., Anderson W. F. Genetic marking shows that Ph+ cells present in autologous transplants of chronic myelogenous leukemia (CML) contribute to relapse after autologous bone marrow in CML. Blood. 1994 May 15;83(10):3068–3076. [PubMed] [Google Scholar]
- Dunphy F. R., Spitzer G., Buzdar A. U., Hortobagyi G. N., Horwitz L. J., Yau J. C., Spinolo J. A., Jagannath S., Holmes F., Wallerstein R. O. Treatment of estrogen receptor-negative or hormonally refractory breast cancer with double high-dose chemotherapy intensification and bone marrow support. J Clin Oncol. 1990 Jul;8(7):1207–1216. doi: 10.1200/JCO.1990.8.7.1207. [DOI] [PubMed] [Google Scholar]
- Engelsman E., Klijn J. C., Rubens R. D., Wildiers J., Beex L. V., Nooij M. A., Rotmensz N., Sylvester R. "Classical" CMF versus a 3-weekly intravenous CMF schedule in postmenopausal patients with advanced breast cancer. An EORTC Breast Cancer Co-operative Group Phase III Trial (10808). Eur J Cancer. 1991;27(8):966–970. doi: 10.1016/0277-5379(91)90259-g. [DOI] [PubMed] [Google Scholar]
- Faucher C., Le Corroller A. G., Chabannon C., Viens P., Stoppa A. M., Bouabdallah R., Camerlo J., Vey N., Gravis G., Gastaut J. A. Autologous transplantation of blood stem cells mobilized with filgrastim alone in 93 patients with malignancies: the number of CD34+ cells reinfused is the only factor predicting both granulocyte and platelet recovery. J Hematother. 1996 Dec;5(6):663–670. doi: 10.1089/scd.1.1996.5.663. [DOI] [PubMed] [Google Scholar]
- Faucher C., le Corroller A. G., Blaise D., Novakovitch G., Manonni P., Moatti J. P., Maraninchi D. Comparison of G-CSF-primed peripheral blood progenitor cells and bone marrow auto transplantation: clinical assessment and cost-effectiveness. Bone Marrow Transplant. 1994 Dec;14(6):895–901. [PubMed] [Google Scholar]
- Fields K. K., Elfenbein G. J., Trudeau W. L., Perkins J. B., Janssen W. E., Moscinski L. C. Clinical significance of bone marrow metastases as detected using the polymerase chain reaction in patients with breast cancer undergoing high-dose chemotherapy and autologous bone marrow transplantation. J Clin Oncol. 1996 Jun;14(6):1868–1876. doi: 10.1200/JCO.1996.14.6.1868. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Antman K., Teicher B., Eder P., Schnipper L. Bone marrow autotransplantation for solid tumors--prospects. J Clin Oncol. 1989 Apr;7(4):515–526. doi: 10.1200/JCO.1989.7.4.515. [DOI] [PubMed] [Google Scholar]
- Gratwohl A., Hermans J., Baldomero H. Hematopoietic precursor cell transplants in Europe: activity in 1994. Report from the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 1996 Feb;17(2):137–148. [PubMed] [Google Scholar]
- Haas R., Möhle R., Frühauf S., Goldschmidt H., Witt B., Flentje M., Wannenmacher M., Hunstein W. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood. 1994 Jun 15;83(12):3787–3794. [PubMed] [Google Scholar]
- Hartmann O., Le Corroller A. G., Blaise D., Michon J., Philip I., Norol F., Janvier M., Pico J. L., Baranzelli M. C., Rubie H. Peripheral blood stem cell and bone marrow transplantation for solid tumors and lymphomas: hematologic recovery and costs. A randomized, controlled trial. Ann Intern Med. 1997 Apr 15;126(8):600–607. doi: 10.7326/0003-4819-126-8-199704150-00002. [DOI] [PubMed] [Google Scholar]
- Hohaus S., Pförsich M., Murea S., Abdallah A., Lin Y. S., Funk L., Voso M. T., Kaul S., Schmid H., Wallwiener D. Immunomagnetic selection of CD34+ peripheral blood stem cells for autografting in patients with breast cancer. Br J Haematol. 1997 Jun;97(4):881–888. doi: 10.1046/j.1365-2141.1997.1272941.x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Beveridge R. A., Rowley S. D., Gordon G. B., Abeloff M. D., Davidson N. E. High-dose chemotherapy with reinfusion of purged autologous bone marrow following dose-intense induction as initial therapy for metastatic breast cancer. J Natl Cancer Inst. 1991 Jul 3;83(13):920–926. doi: 10.1093/jnci/83.13.920. [DOI] [PubMed] [Google Scholar]
- Lemoli R. M., Fortuna A., Motta M. R., Rizzi S., Giudice V., Nannetti A., Martinelli G., Cavo M., Amabile M., Mangianti S. Concomitant mobilization of plasma cells and hematopoietic progenitors into peripheral blood of multiple myeloma patients: positive selection and transplantation of enriched CD34+ cells to remove circulating tumor cells. Blood. 1996 Feb 15;87(4):1625–1634. [PubMed] [Google Scholar]
- Link H., Arseniev L., Bähre O., Kadar J. G., Diedrich H., Poliwoda H. Transplantation of allogeneic CD34+ blood cells. Blood. 1996 Jun 1;87(11):4903–4909. [PubMed] [Google Scholar]
- Lopez M., Lemoine F. M., Firat H., Fouillard L., Laporte J. P., Lesage S., Isnard F., Stachowiak J., Ferrer-Le Coeur F., Morel P. Bone marrow versus peripheral blood progenitor cells CD34 selection in patients with non-Hodgkin's lymphomas: different levels of tumor cell reduction. Implications for autografting. Blood. 1997 Oct 1;90(7):2830–2838. [PubMed] [Google Scholar]
- Mahé B., Milpied N., Hermouet S., Robillard N., Moreau P., Letortorec S., Rapp M. J., Bataille R., Harousseau J. L. G-CSF alone mobilizes sufficient peripheral blood CD34+ cells for positive selection in newly diagnosed patients with myeloma. Br J Haematol. 1996 Feb;92(2):263–268. doi: 10.1046/j.1365-2141.1996.d01-1506.x. [DOI] [PubMed] [Google Scholar]
- Mapara M. Y., Körner I. J., Hildebrandt M., Bargou R., Krahl D., Reichardt P., Dörken B. Monitoring of tumor cell purging after highly efficient immunomagnetic selection of CD34 cells from leukapheresis products in breast cancer patients: comparison of immunocytochemical tumor cell staining and reverse transcriptase-polymerase chain reaction. Blood. 1997 Jan 1;89(1):337–344. [PubMed] [Google Scholar]
- Marin G. H., Dal Cortivo L., Cayuela J. M., Marolleau J. P., Pautier P., Cojean-Zelek I., Brice P., Makke J., Benbunan M., Gisselbrecht C. Peripheral blood stem cell CD34+ autologous transplant in relapsed follicular lymphoma. Hematol Cell Ther. 1997 Feb;39(1):33–40. doi: 10.1007/s00282-997-0033-4. [DOI] [PubMed] [Google Scholar]
- McQuaker I. G., Haynes A. P., Anderson S., Stainer C., Owen R. G., Morgan G. J., Lumley M., Milligan D., Fletcher J., Bessell E. M. Engraftment and molecular monitoring of CD34+ peripheral-blood stem-cell transplants for follicular lymphoma: a pilot study. J Clin Oncol. 1997 Jun;15(6):2288–2295. doi: 10.1200/JCO.1997.15.6.2288. [DOI] [PubMed] [Google Scholar]
- Moss T. J., Ross A. A. The risk of tumor cell contamination in peripheral blood stem cell collections. J Hematother. 1992 Fall;1(3):225–232. doi: 10.1089/scd.1.1992.1.225. [DOI] [PubMed] [Google Scholar]
- Peters W. P., Ross M., Vredenburgh J. J., Meisenberg B., Marks L. B., Winer E., Kurtzberg J., Bast R. C., Jr, Jones R., Shpall E. High-dose chemotherapy and autologous bone marrow support as consolidation after standard-dose adjuvant therapy for high-risk primary breast cancer. J Clin Oncol. 1993 Jun;11(6):1132–1143. doi: 10.1200/JCO.1993.11.6.1132. [DOI] [PubMed] [Google Scholar]
- Ross A. A., Cooper B. W., Lazarus H. M., Mackay W., Moss T. J., Ciobanu N., Tallman M. S., Kennedy M. J., Davidson N. E., Sweet D. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood. 1993 Nov 1;82(9):2605–2610. [PubMed] [Google Scholar]
- Schmitz N., Linch D. C., Dreger P., Goldstone A. H., Boogaerts M. A., Ferrant A., Demuynck H. M., Link H., Zander A., Barge A. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996 Feb 10;347(8998):353–357. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- Shpall E. J., Bast R. C., Jr, Joines W. T., Jones R. B., Anderson I., Johnston C., Eggleston S., Tepperberg M., Edwards S., Peters W. P. Immunomagnetic purging of breast cancer from bone marrow for autologous transplantation. Bone Marrow Transplant. 1991 Feb;7(2):145–151. [PubMed] [Google Scholar]
- Shpall E. J., Jones R. B., Bast R. C., Jr, Rosner G. L., Vandermark R., Ross M., Affronti M. L., Johnston C., Eggleston S., Tepperburg M. 4-Hydroperoxycyclophosphamide purging of breast cancer from the mononuclear cell fraction of bone marrow in patients receiving high-dose chemotherapy and autologous marrow support: a phase I trial. J Clin Oncol. 1991 Jan;9(1):85–93. doi: 10.1200/JCO.1991.9.1.85. [DOI] [PubMed] [Google Scholar]
- Shpall E. J., Jones R. B., Bearman S. I., Franklin W. A., Archer P. G., Curiel T., Bitter M., Claman H. N., Stemmer S. M., Purdy M. Transplantation of enriched CD34-positive autologous marrow into breast cancer patients following high-dose chemotherapy: influence of CD34-positive peripheral-blood progenitors and growth factors on engraftment. J Clin Oncol. 1994 Jan;12(1):28–36. doi: 10.1200/JCO.1994.12.1.28. [DOI] [PubMed] [Google Scholar]
- Shpall E. J., LeMaistre C. F., Holland K., Ball E., Jones R. B., Saral R., Jacobs C., Heimfeld S., Berenson R., Champlin R. A prospective randomized trial of buffy coat versus CD34-selected autologous bone marrow support in high-risk breast cancer patients receiving high-dose chemotherapy. Blood. 1997 Dec 1;90(11):4313–4320. [PubMed] [Google Scholar]
- Simmons D. L., Satterthwaite A. B., Tenen D. G., Seed B. Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol. 1992 Jan 1;148(1):267–271. [PubMed] [Google Scholar]
- Somlo G., Sniecinski I., Odom-Maryon T., Nowicki B., Chow W., Hamasaki V., Leong L., Margolin K., Morgan R., Jr, Raschko J. Effect of CD34+ selection and various schedules of stem cell reinfusion and granulocyte colony-stimulating factor priming on hematopoietic recovery after high-dose chemotherapy for breast cancer. Blood. 1997 Mar 1;89(5):1521–1528. [PubMed] [Google Scholar]
- Tannock I. F., Boyd N. F., DeBoer G., Erlichman C., Fine S., Larocque G., Mayers C., Perrault D., Sutherland H. A randomized trial of two dose levels of cyclophosphamide, methotrexate, and fluorouracil chemotherapy for patients with metastatic breast cancer. J Clin Oncol. 1988 Sep;6(9):1377–1387. doi: 10.1200/JCO.1988.6.9.1377. [DOI] [PubMed] [Google Scholar]
- To L. B., Haylock D. N., Simmons P. J., Juttner C. A. The biology and clinical uses of blood stem cells. Blood. 1997 Apr 1;89(7):2233–2258. [PubMed] [Google Scholar]
- Weaver C. H., Hazelton B., Birch R., Palmer P., Allen C., Schwartzberg L., West W. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995 Nov 15;86(10):3961–3969. [PubMed] [Google Scholar]
- Williams S. F., Lee W. J., Bender J. G., Zimmerman T., Swinney P., Blake M., Carreon J., Schilling M., Smith S., Williams D. E. Selection and expansion of peripheral blood CD34+ cells in autologous stem cell transplantation for breast cancer. Blood. 1996 Mar 1;87(5):1687–1691. [PubMed] [Google Scholar]
- van der Wall E., Richel D. J., Holtkamp M. J., Slaper-Cortenbach I. C., van der Schoot C. E., Dalesio O., Nooijen W. J., Schornagel J. H., Rodenhuis S. Bone marrow reconstitution after high-dose chemotherapy and autologous peripheral blood progenitor cell transplantation: effect of graft size. Ann Oncol. 1994 Nov;5(9):795–802. doi: 10.1093/oxfordjournals.annonc.a059007. [DOI] [PubMed] [Google Scholar]


