Abstract
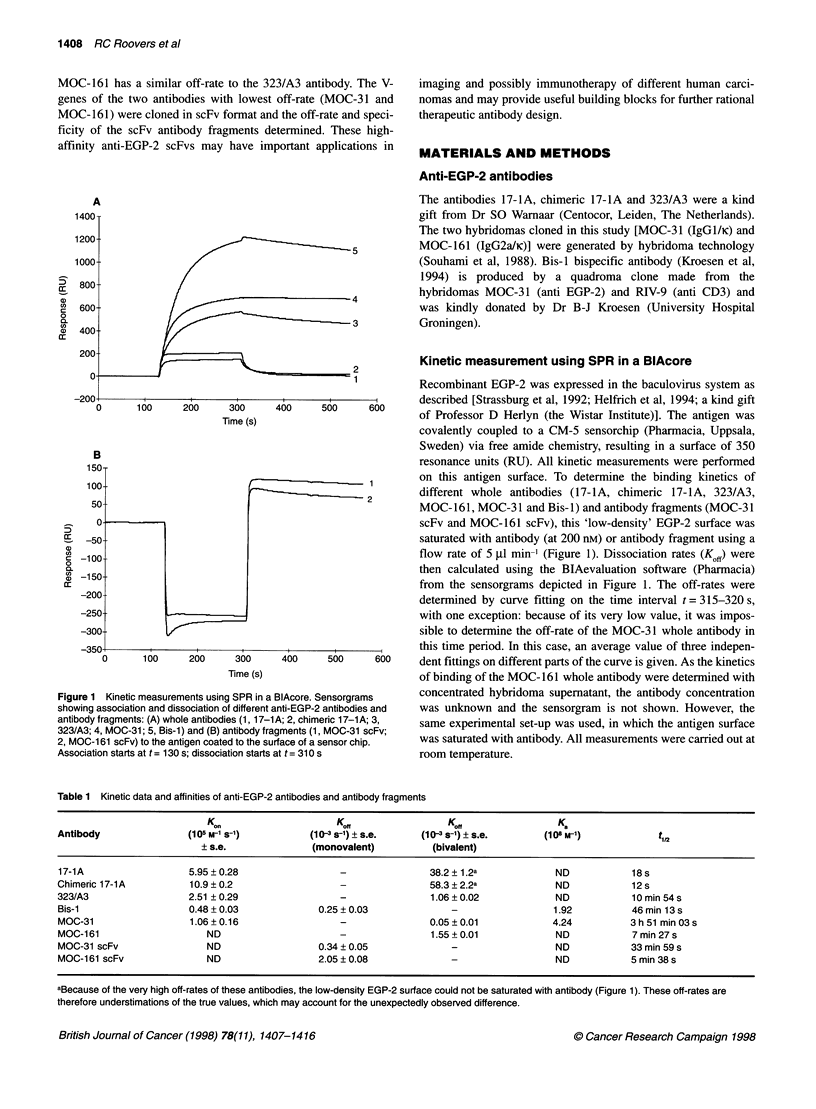
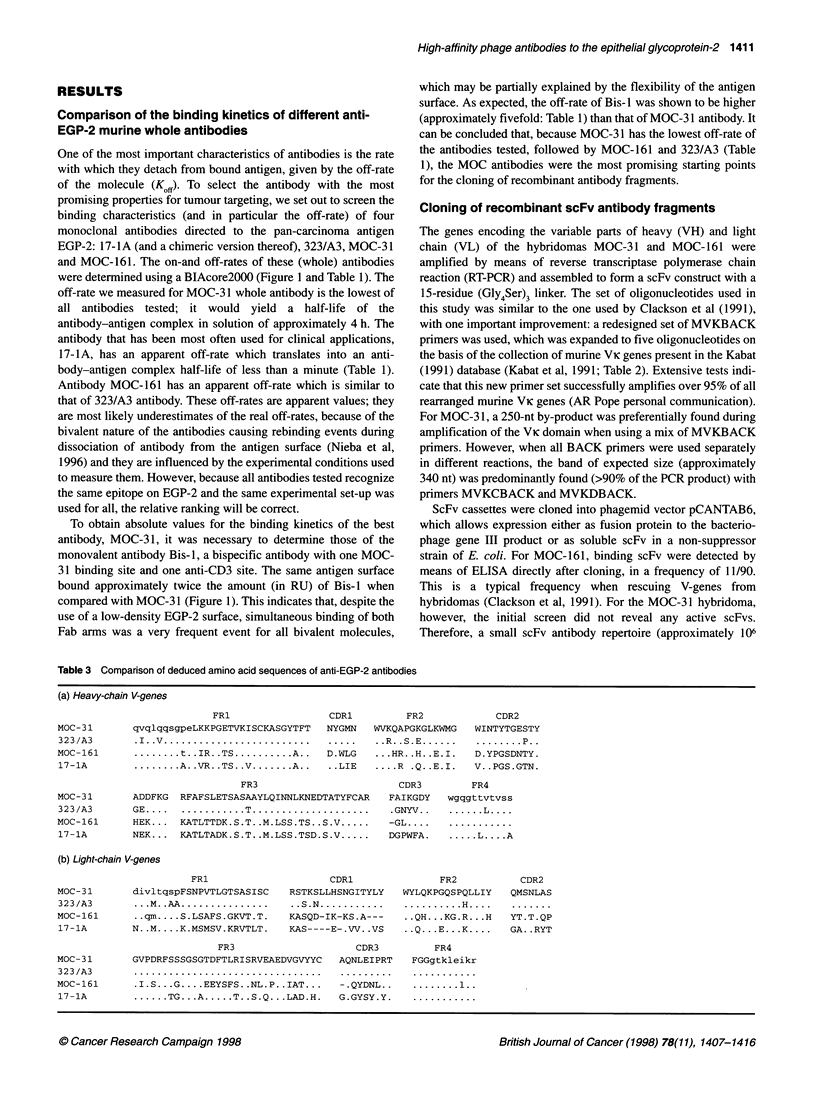
The tumour-associated antigen epithelial glycoprotein-2 (EGP-2) is a promising target for detection and treatment of a variety of human carcinomas. Antibodies to this antigen have been successfully used in patients for imaging of small-cell lung cancer and for adjuvant treatment of minimal residual disease of colon cancer. We describe here the isolation and complete characterization of high-affinity single-chain variable fragments (scFv) to the EGP-2 antigen. First, the binding kinetics of four murine whole antibodies directed to EGP-2 (17-1A, 323/A3, MOC-31 and MOC-161) were determined using surface plasmon resonance (SPR). The MOC-31 antibody has the lowest apparent off-rate, followed by MOC-161 and 323/A3. The V-genes of the two MOC hybridomas were cloned as scFv in a phage display vector and antigen-binding phage were selected by panning on recombinant antigen. The scFvs compete with the original hybridoma antibodies for binding to antigen and specifically bind to human carcinomas in immunohistochemistry. MOC-31 scFv has an off-rate which is better than those of the bivalent 17-1A and 323/A3 whole antibodies, providing it with an essential characteristic for tumour retention in vivo. The availability of these high-affinity anti-EGP-2 antibody fragments and of their encoding V-genes creates a variety of possibilities for their future use as tumour-targeting vehicles.
Full text
PDF


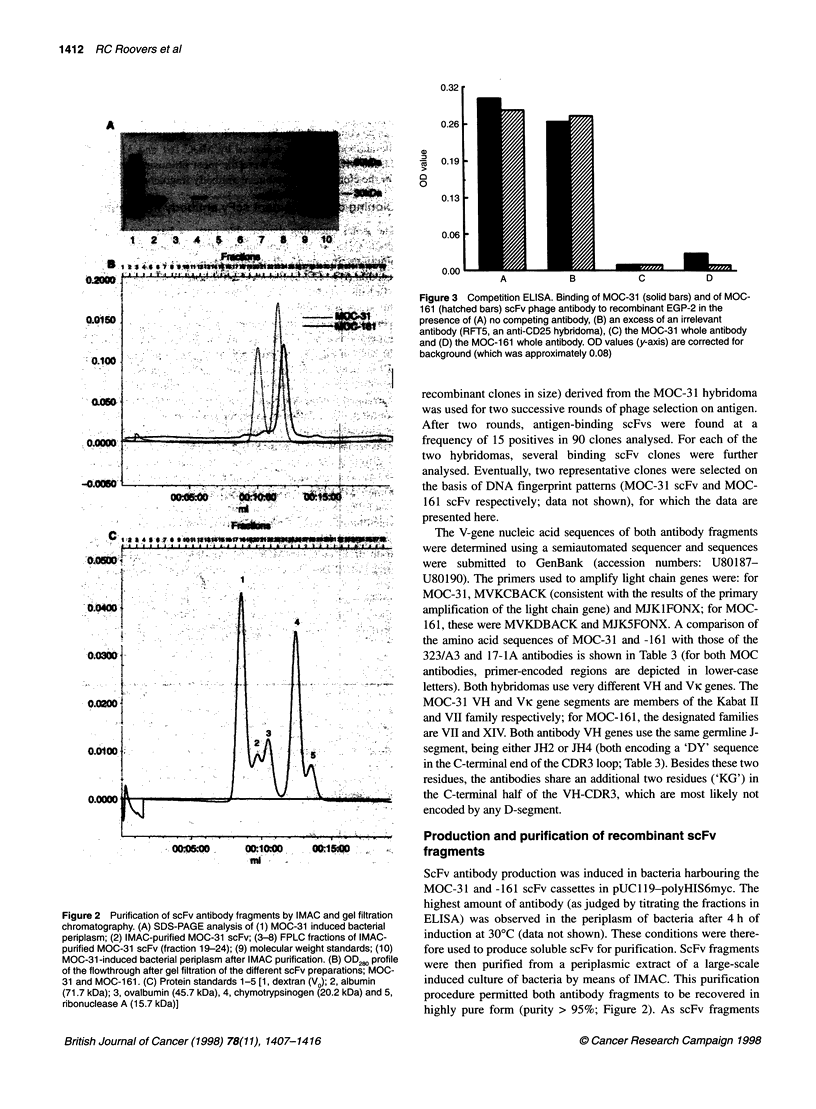
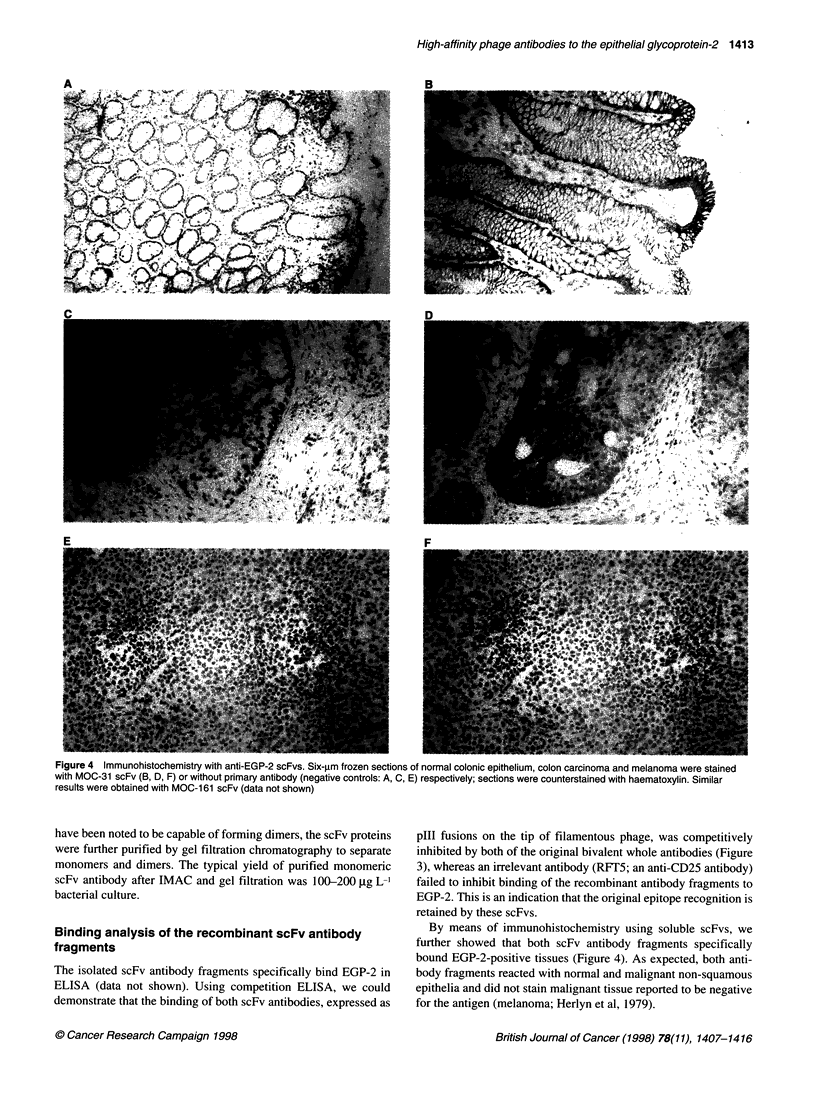



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. P., McCartney J. E., Tai M. S., Oppermann H., Huston J. S., Stafford W. F., 3rd, Bookman M. A., Fand I., Houston L. L., Weiner L. M. Highly specific in vivo tumor targeting by monovalent and divalent forms of 741F8 anti-c-erbB-2 single-chain Fv. Cancer Res. 1993 Sep 1;53(17):4026–4034. [PubMed] [Google Scholar]
- Begent R. H., Verhaar M. J., Chester K. A., Casey J. L., Green A. J., Napier M. P., Hope-Stone L. D., Cushen N., Keep P. A., Johnson C. J. Clinical evidence of efficient tumor targeting based on single-chain Fv antibody selected from a combinatorial library. Nat Med. 1996 Sep;2(9):979–984. doi: 10.1038/nm0996-979. [DOI] [PubMed] [Google Scholar]
- Clackson T., Hoogenboom H. R., Griffiths A. D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991 Aug 15;352(6336):624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Abrahmsén L., Björk P., Lando P. A., Hedlund G., Forsberg G., Brodin T., Gascoigne N. R., Förberg C., Lind P. Monoclonal antibody-superantigen fusion proteins: tumor-specific agents for T-cell-based tumor therapy. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8945–8949. doi: 10.1073/pnas.91.19.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. P., Grzyb K. T., Dressler L. G., Mansel R. E., Zava D. T., Sledge G. W., Jr, McGuire W. L. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986 Mar;46(3):1306–1317. [PubMed] [Google Scholar]
- Fagerberg J., Steinitz M., Wigzell H., Askelöf P., Mellstedt H. Human anti-idiotypic antibodies induced a humoral and cellular immune response against a colorectal carcinoma-associated antigen in patients. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4773–4777. doi: 10.1073/pnas.92.11.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frödin J. E., Harmenberg U., Biberfeld P., Christensson B., Lefvert A. K., Rieger A., Shetye J., Wahren B., Mellstedt H. Clinical effects of monoclonal antibodies (MAb 17-1A) in patients with metastatic colorectal carcinomas. Hybridoma. 1988 Aug;7(4):309–321. doi: 10.1089/hyb.1988.7.309. [DOI] [PubMed] [Google Scholar]
- Griffiths A. D., Williams S. C., Hartley O., Tomlinson I. M., Waterhouse P., Crosby W. L., Kontermann R. E., Jones P. T., Low N. M., Allison T. J. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 1994 Jul 15;13(14):3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich W., Kroesen B. J., Roovers R. C., Westers L., Molema G., Hoogenboom H. R., de Leij L. Construction and characterization of a bispecific diabody for retargeting T cells to human carcinomas. Int J Cancer. 1998 Apr 13;76(2):232–239. doi: 10.1002/(sici)1097-0215(19980413)76:2<232::aid-ijc11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Helfrich W., Van Geel M., The T. H., De Leij L. Detection of a putative 30-kDa ligand of the cluster-2 antigen. Int J Cancer Suppl. 1994;8:70–75. doi: 10.1002/ijc.2910570715. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. CO 17-1A and related monoclonal antibodies: their production and characterization. Hybridoma. 1986 Jul;5 (Suppl 1):S3–10. [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1438–1442. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom H. R., Griffiths A. D., Johnson K. S., Chiswell D. J., Hudson P., Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991 Aug 11;19(15):4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespers L. S., Roberts A., Mahler S. M., Winter G., Hoogenboom H. R. Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen. Biotechnology (N Y) 1994 Sep;12(9):899–903. doi: 10.1038/nbt0994-899. [DOI] [PubMed] [Google Scholar]
- Kortt A. A., Lah M., Oddie G. W., Gruen C. L., Burns J. E., Pearce L. A., Atwell J. L., McCoy A. J., Howlett G. J., Metzger D. W. Single-chain Fv fragments of anti-neuraminidase antibody NC10 containing five- and ten-residue linkers form dimers and with zero-residue linker a trimer. Protein Eng. 1997 Apr;10(4):423–433. doi: 10.1093/protein/10.4.423. [DOI] [PubMed] [Google Scholar]
- Kosterink J. G., de Jonge M. W., Smit E. F., Piers D. A., Kengen R. A., Postmus P. E., Shochat D., Groen H. J., The H. T., de Leij L. Pharmacokinetics and scintigraphy of indium-111-DTPA-MOC-31 in small-cell lung carcinoma. J Nucl Med. 1995 Dec;36(12):2356–2362. [PubMed] [Google Scholar]
- Krebber A., Bornhauser S., Burmester J., Honegger A., Willuda J., Bosshard H. R., Plückthun A. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J Immunol Methods. 1997 Feb 14;201(1):35–55. doi: 10.1016/s0022-1759(96)00208-6. [DOI] [PubMed] [Google Scholar]
- Kroesen B. J., Buter J., Sleijfer D. T., Janssen R. A., van der Graaf W. T., The T. H., de Leij L., Mulder N. H. Phase I study of intravenously applied bispecific antibody in renal cell cancer patients receiving subcutaneous interleukin 2. Br J Cancer. 1994 Oct;70(4):652–661. doi: 10.1038/bjc.1994.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaistre C. F., Edwards D. P., Krolick K. A., McGuire W. L. An immunotoxin cytotoxic for breast cancer cells in vitro. Cancer Res. 1987 Feb 1;47(3):730–734. [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- Nieba L., Krebber A., Plückthun A. Competition BIAcore for measuring true affinities: large differences from values determined from binding kinetics. Anal Biochem. 1996 Feb 15;234(2):155–165. doi: 10.1006/abio.1996.0067. [DOI] [PubMed] [Google Scholar]
- Riethmüller G., Schneider-Gädicke E., Schlimok G., Schmiegel W., Raab R., Höffken K., Gruber R., Pichlmaier H., Hirche H., Pichlmayr R. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes' C colorectal carcinoma. German Cancer Aid 17-1A Study Group. Lancet. 1994 May 14;343(8907):1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- Schier R., Bye J., Apell G., McCall A., Adams G. P., Malmqvist M., Weiner L. M., Marks J. D. Isolation of high-affinity monomeric human anti-c-erbB-2 single chain Fv using affinity-driven selection. J Mol Biol. 1996 Jan 12;255(1):28–43. doi: 10.1006/jmbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- Strassburg C. P., Kasai Y., Seng B. A., Miniou P., Zaloudik J., Herlyn D., Koprowski H., Linnenbach A. J. Baculovirus recombinant expressing a secreted form of a transmembrane carcinoma-associated antigen. Cancer Res. 1992 Feb 15;52(4):815–821. [PubMed] [Google Scholar]
- Tai M. S., McCartney J. E., Adams G. P., Jin D., Hudziak R. M., Oppermann H., Laminet A. A., Bookman M. A., Wolf E. J., Liu S. Targeting c-erbB-2 expressing tumors using single-chain Fv monomers and dimers. Cancer Res. 1995 Dec 1;55(23 Suppl):5983s–5989s. [PubMed] [Google Scholar]
- Velders M. P., Litvinov S. V., Warnaar S. O., Gorter A., Fleuren G. J., Zurawski V. R., Jr, Coney L. R. New chimeric anti-pancarcinoma monoclonal antibody with superior cytotoxicity-mediating potency. Cancer Res. 1994 Apr 1;54(7):1753–1759. [PubMed] [Google Scholar]
- Velders M. P., van Rhijn C. M., Briaire I. H., Fleuren G. J., Warnaar S. O., Litvinov S. V. Immunotherapy with low and high affinity monoclonal antibodies 17-1A and 323/A3 in a nude mouse xenograft carcinoma model. Cancer Res. 1995 Oct 1;55(19):4398–4403. [PubMed] [Google Scholar]
- Winter G., Griffiths A. D., Hawkins R. E., Hoogenboom H. R. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Chen W., Raubitschek A., Williams L. E., Neumaier M., Fischer R., Hu S. Z., Odom-Maryon T., Wong J. Y., Shively J. E. Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology. 1996 Feb;2(1):21–36. doi: 10.1016/1380-2933(95)00027-5. [DOI] [PubMed] [Google Scholar]
- Zimmermann S., Wels W., Froesch B. A., Gerstmayer B., Stahel R. A., Zangemeister-Wittke U. A novel immunotoxin recognising the epithelial glycoprotein-2 has potent antitumoural activity on chemotherapy-resistant lung cancer. Cancer Immunol Immunother. 1997 Mar;44(1):1–9. doi: 10.1007/s002620050348. [DOI] [PMC free article] [PubMed] [Google Scholar]