Abstract
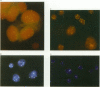
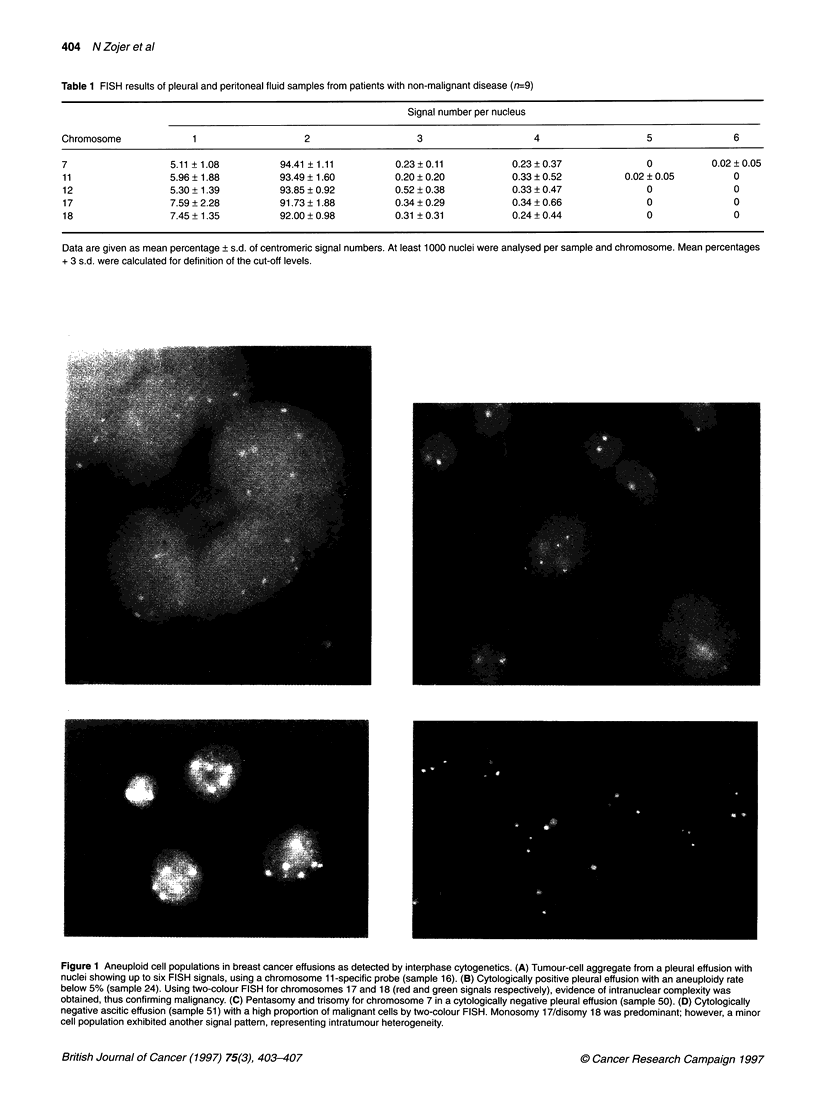
In diagnostic evaluation of effusions, difficulties are encountered when atypical reactive mesothelial cells have to be differentiated from malignant cells. We tested the impact of fluorescence in situ hybridization (FISH) to identify metastatic cells in breast cancer effusions by detection of numerical chromosomal changes. Pleural and ascitic fluid samples (n=57) from 41 breast cancer patients were concomitantly evaluated by routine cytology and FISH, using centromere-specific probes representing chromosomes 7, 11, 12, 17 and 18. After setting stringent cut-off levels deduced from non-malignant control effusions (n=9), the rates of cells with true aneuploidy were determined in each effusion sample from breast cancer patients. The occurrence of aneuploid cells, as detected by FISH and indicative of malignancy, was correlated with the cytological findings. Routine cytology revealed malignancy in 60% of effusions. Using FISH, aneuploid cell populations could be observed in 94% of cytologically positive and in 48% of cytologically negative effusions, thus reverting diagnosis to malignancy. To confirm malignancy in cases with a low frequency of aneuploid cells, two-colour FISH was additionally performed and indeed showed heterogeneous chromosomal aneuploidy within single nuclei. We conclude that FISH is a valuable tool in the diagnosis of malignancy and may serve as an adjunct to routine cytological examination, as demonstrated here for breast cancer effusions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athanassiadou P., Athanassiades P., Lazaris D., Kyrkou K., Petrakakou E., Aravantinos D. Immunocytochemical differentiation of reactive mesothelial cells and adenocarcinoma cells in serous effusions with the use of carcinoembryonic antigen and fibronectin. Acta Cytol. 1994 Sep-Oct;38(5):718–722. [PubMed] [Google Scholar]
- Bandyk M. G., Zhao L., Troncoso P., Pisters L. L., Palmer J. L., von Eschenbach A. C., Chung L. W., Liang J. C. Trisomy 7: a potential cytogenetic marker of human prostate cancer progression. Genes Chromosomes Cancer. 1994 Jan;9(1):19–27. doi: 10.1002/gcc.2870090105. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Willetts I., Robertson J. F., Blamey R. W. Pleural effusion in breast cancer: a review of the Nottingham experience. Eur J Surg Oncol. 1994 Feb;20(1):33–36. [PubMed] [Google Scholar]
- Beerman H., Smit V. T., Kluin P. M., Bonsing B. A., Hermans J., Cornelisse C. J. Flow cytometric analysis of DNA stemline heterogeneity in primary and metastatic breast cancer. Cytometry. 1991;12(2):147–154. doi: 10.1002/cyto.990120208. [DOI] [PubMed] [Google Scholar]
- Bentz M., Schröder M., Herz M., Stilgenbauer S., Lichter P., Döhner H. Detection of trisomy 8 on blood smears using fluorescence in situ hybridization. Leukemia. 1993 May;7(5):752–757. [PubMed] [Google Scholar]
- Biesterfeld S., Gerres K., Fischer-Wein G., Böcking A. Polyploidy in non-neoplastic tissues. J Clin Pathol. 1994 Jan;47(1):38–42. doi: 10.1136/jcp.47.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield L. R., Greenberg M. L., Pacey F. Cytogenetic diagnosis of cancer from body fluids. Acta Cytol. 1985 Sep-Oct;29(5):768–774. [PubMed] [Google Scholar]
- Casalone R., Minelli E., Righi R., Granata P., Meroni E., Caruso V., Mazzola D., Salvadore M., Pozzi E., Bono A. V. Clonal chromosome changes in non-neoplastic ureters. Cancer Genet Cytogenet. 1995 Aug;83(1):28–31. doi: 10.1016/s0165-4608(95)00015-1. [DOI] [PubMed] [Google Scholar]
- De Angelis M., Buley I. D., Heryet A., Gray W. Immunocytochemical staining of serous effusions with the monoclonal antibody Ber-EP4. Cytopathology. 1992;3(2):111–117. doi: 10.1111/j.1365-2303.1992.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Drach J., Schuster J., Nowotny H., Angerler J., Rosenthal F., Fiegl M., Rothermundt C., Gsur A., Jäger U., Heinz R. Multiple myeloma: high incidence of chromosomal aneuploidy as detected by interphase fluorescence in situ hybridization. Cancer Res. 1995 Sep 1;55(17):3854–3859. [PubMed] [Google Scholar]
- Eastmond D. A., Schuler M., Rupa D. S. Advantages and limitations of using fluorescence in situ hybridization for the detection of aneuploidy in interphase human cells. Mutat Res. 1995 Dec;348(4):153–162. doi: 10.1016/0165-7992(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Escudier S. M., Pereira-Leahy J. M., Drach J. W., Weier H. U., Goodacre A. M., Cork M. A., Trujillo J. M., Keating M. J., Andreeff M. Fluorescent in situ hybridization and cytogenetic studies of trisomy 12 in chronic lymphocytic leukemia. Blood. 1993 May 15;81(10):2702–2707. [PubMed] [Google Scholar]
- Fiegl M., Tueni C., Schenk T., Jakesz R., Gnant M., Reiner A., Rudas M., Pirc-Danoewinata H., Marosi C., Huber H. Interphase cytogenetics reveals a high incidence of aneuploidy and intra-tumour heterogeneity in breast cancer. Br J Cancer. 1995 Jul;72(1):51–55. doi: 10.1038/bjc.1995.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioanni J., Caldani C., Zanghellini E., Mazeau C., Duplay H., Ferrua B., Schneider M. A new epithelial membrane antigen (Calam 27) as a marker of carcinoma in serous effusions. Acta Cytol. 1991 May-Jun;35(3):315–319. [PubMed] [Google Scholar]
- Heim S., Mandahl N., Mitelman F. Genetic convergence and divergence in tumor progression. Cancer Res. 1988 Nov 1;48(21):5911–5916. [PubMed] [Google Scholar]
- Herrington C. S., Cooper K., McGee J. O. Interphase cytogenetics: analysis of numerical chromosome aberrations in isolated cells. J Pathol. 1995 Mar;175(3):283–295. doi: 10.1002/path.1711750306. [DOI] [PubMed] [Google Scholar]
- Hopman A. H., Ramaekers F. C., Raap A. K., Beck J. L., Devilee P., van der Ploeg M., Vooijs G. P. In situ hybridization as a tool to study numerical chromosome aberrations in solid bladder tumors. Histochemistry. 1988;89(4):307–316. doi: 10.1007/BF00500631. [DOI] [PubMed] [Google Scholar]
- Illingworth A. L., Young J. A., Johnson G. D. Immunofluorescent staining of metastatic carcinoma cells in serious fluid with carcinoembryonic antibody, epithelial membrane antibody, AUA-1 and Ber-EP4. Cytopathology. 1994 Oct;5(5):270–281. doi: 10.1111/j.1365-2303.1994.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Johnston W. W. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985 Aug 15;56(4):905–909. doi: 10.1002/1097-0142(19850815)56:4<905::aid-cncr2820560435>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Joseph M. G., Banerjee D., Harris P., Gibson S., McFadden R. G. Multiparameter flow cytometric DNA analysis of effusions: a prospective study of 36 cases compared with routine cytology and immunohistochemistry. Mod Pathol. 1995 Aug;8(6):686–693. [PubMed] [Google Scholar]
- Kibbelaar R. E., Kok F., Dreef E. J., Kleiverda J. K., Cornelisse C. J., Raap A. K., Kluin P. M. Statistical methods in interphase cytogenetics: an experimental approach. Cytometry. 1993 Oct;14(7):716–724. doi: 10.1002/cyto.990140704. [DOI] [PubMed] [Google Scholar]
- LEUALLEN E. C., CARR D. T. Pleural effusion; a statistical study of 436 patients. N Engl J Med. 1955 Jan 20;252(3):79–83. doi: 10.1056/NEJM195501202520301. [DOI] [PubMed] [Google Scholar]
- Osinaga E., Pancino G., Beuzelin M., Babino A., Rodriguez D., Robello C., Tiscornia A., Phillips E., Bourguignat A., Roseto A. Detection of a soluble antigen defined by monoclonal antibody 83D4 in serous effusions associated with breast carcinoma. Cancer. 1992 Apr 1;69(7):1745–1749. doi: 10.1002/1097-0142(19920401)69:7<1745::aid-cncr2820690716>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Starr R. L., Sherman M. E. The value of multiple preparations in the diagnosis of malignant pleural effusions. A cost-benefit analysis. Acta Cytol. 1991 Sep-Oct;35(5):533–537. [PubMed] [Google Scholar]
- Tanner M. M., Tirkkonen M., Kallioniemi A., Holli K., Collins C., Kowbel D., Gray J. W., Kallioniemi O. P., Isola J. Amplification of chromosomal region 20q13 in invasive breast cancer: prognostic implications. Clin Cancer Res. 1995 Dec;1(12):1455–1461. [PubMed] [Google Scholar]
- Taylor C., Patel K., Jones T., Kiely F., De Stavola B. L., Sheer D. Diagnosis of Ewing's sarcoma and peripheral neuroectodermal tumour based on the detection of t(11;22) using fluorescence in situ hybridisation. Br J Cancer. 1993 Jan;67(1):128–133. doi: 10.1038/bjc.1993.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M. R., Pandis N., Bardi G., Andersen J. A., Mandahl N., Mitelman F., Heim S. Cytogenetic analysis of multifocal breast carcinomas: detection of karyotypically unrelated clones as well as clonal similarities between tumour foci. Br J Cancer. 1994 Nov;70(5):922–927. doi: 10.1038/bjc.1994.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J., Yang J. M., Emerson J., Dalton W., McGee D., Massey K., Thompson F., Villar H. Clonal chromosome abnormalities in human breast carcinomas. II. Thirty-four cases with metastatic disease. Genes Chromosomes Cancer. 1993 Aug;7(4):194–203. doi: 10.1002/gcc.2870070403. [DOI] [PubMed] [Google Scholar]