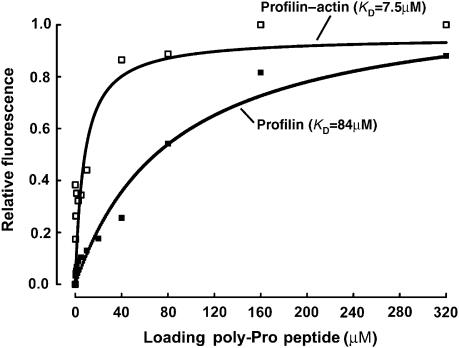
Figure 2.
Binding of profilin and profilin–actin to the loading poly-Pro site of VASP. Binding of the VASP peptide (198GAGGGPPP APPLPAAQ213) produces a significant change in the intrinsic tryptophan fluorescence of profilin alone (▪) and profilin–actin (□). In this experiment, the concentration of profilin and profilin–actin was 5 μM and the concentration of the VASP peptide varied for 0–320 μM (see Materials and methods). Each data point corresponds to the average of five independent measurements. Least-square fitting of the data, using a single-site binding model, resulted in dissociation constant (KD) estimates of 84 and 7.5 μM for profilin and profilin–actin, respectively.