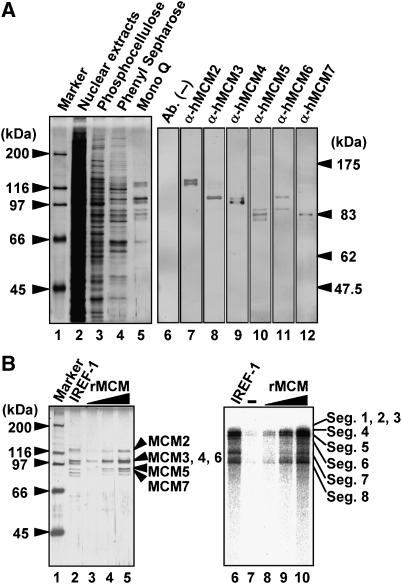
Figure 3.
Identification of functional components in IREF-1. (A) (Left panel) SDS–PAGE analysis. The loaded amounts were adjusted to the equal level of the IREF-1 stimulatory activity attained in the cell-free virus genome replication assay. Lane 1, molecular size marker (Bio-Rad); lane 2, uninfected HeLa cell nuclear extracts; lane 3, 0.2 M KCl eluate from phosphocellulose column; lane 4, 0.25 M (NH4)2SO4 eluate from phenyl Sepharose column; lane 5, 0.33 M KCl eluate from Mono Q column (purified IREF-1 fraction). The gel was visualized by silver staining assay. (Right panel) Western blotting assay. The IREF-1 fraction of Mono Q column was separated through SDS–PAGE, and subjected to western blotting assays with rabbit anti-MCM2, 3, 4, 5, 6, and 7 antibodies. (B) (Left panel) Purification of recombinant MCM complex from insect cells. The details of purification scheme and column chromatography are described in Supplementary data. Lane 1, molecular size marker (Bio-Rad); lane 2, purified IREF-1 fraction from uninfected HeLa nuclear extracts (Mono Q fraction); lanes 3–5, recombinant MCM heterohexamer complex containing 7.5 (lane 3), 15 (lane 4), 30 ng (lane 5) of MCM2 equivalent. The gel was visualized by silver staining assay. (Right panel) Cell-free virus genome replication assay. Equal amounts of IREF-1 and rMCM loaded in left panel were examined in the cell-free virus genome replication assay. Lane 6, purified IREF-1 fraction (Mono Q fraction); lanes 8–10, rMCM complex containing 7.5, 15, 30 ng of rMCM2. The assay in lane 7 was carried out in the absence of any proteins.