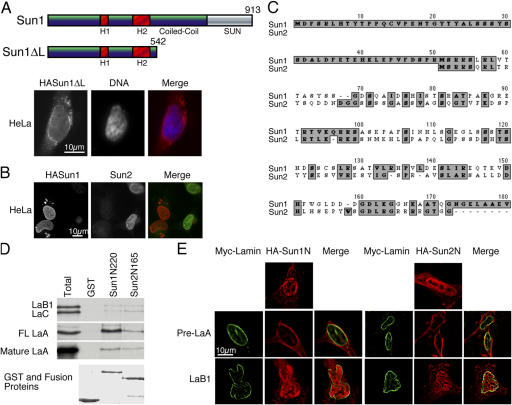
Figure 4.
Interactions between the nucleoplasmic domains of Sun1 and 2 with A-type lamins. (A) An HA-Sun1ΔL construct lacking the SUN domain and most of the lumenal coiled-coil localizes to the NE in a manner similar to the full-length protein. Anti-HA labeling is in red, whereas DNA, visualized with Höchst dye, is in blue. (B) Overexpression of HA-Sun1 leads to the loss of endogenous Sun2 in HeLa cells, suggesting that these two proteins share common binding partners. (C) A ClustalW alignment of the NH2-terminal sequences of mouse Sun1 and 2 identifies multiple clusters of homologous amino acids within a region of ∼120 residues. Sun1 exhibits a unique NH2-terminal extension of 50 amino acid residues. (D) The first 222 residues of Sun1 or the first 165 residues of Sun2 (this represents the entire nucleoplasmic domain of the latter) were fused to GST and, with GST alone (Coomassie stain, bottom), were used to pull down 35S-labeled, in vitro translated lamins B1, C, mature A, and full-length (FL) A (Total). Unlike GST alone, Sun1N220 and Sun2N165 pulled down lamins B1, C, and mature A at similar levels. However, Sun1N222 displayed a higher affinity for FL LaA than did Sun2N165. Evidently, Sun1 has a very strong preference for full-length (or pre) lamin A over mature lamin A (or full-length lamins C and B1). (E) An HA tag was inserted at the NH2 terminus of the same nucleoplasmic segments of both Sun1 and 2 (as described in D), and the tagged proteins were expressed in HeLa cells. Both of these exogenous proteins appear enriched in the nucleoplasm (top). As observed by deconvolution microscopy, cotransfection of the myc-tagged full-length lamin A (green) with HA-Sun1N222 or HA-Sun2N165 (red) resulted in the recruitment of both HA-Sun proteins to the NE (middle). Myc–lamin B1, in contrast, fails to produce such an effect. Evidently, the nucleoplasmic domains of Sun1 and 2 can interact with lamin A in vivo.