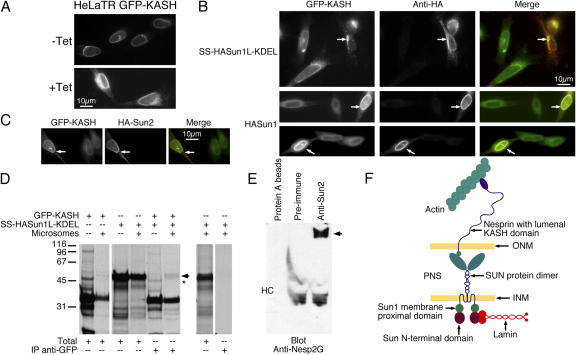
Figure 8.
The nesp2G KASH domain interacts with the Sun1 lumenal domain. (A) Fluorescence microscopy of HeLa cells expressing a tetracycline-inducible GFP-KASH fusion protein (HeLaTR GFP-KASH). In the absence of tetracycline (−Tet), GFP-KASH is expressed at low levels and is localized exclusively to the NE. After induction with tetracycline for 24 h (+Tet), GFP-KASH is found throughout the peripheral ER as well as the NE. Expression levels of GFP-KASH within the cell population is extremely uniform both before and after induction. (B) Transfection of SS–HA-Sun1–KDEL into the HeLaTR GFP-KASH cells after tetracycline induction resulted in the complete loss of GFP-KASH from the NE. This was accompanied by the formation of cytoplasmic aggregates (arrows, top). Conversely, introduction of full-length HA-Sun1 into these cells resulted in the recruitment of GFP-KASH to the NE (arrows, middle and bottom). (C) HA-Sun2 was found to have a similar effect (arrows). In the merged images in B and C, GFP-KASH is presented in green, whereas HA-Sun is shown in red. (D) To identify an in vitro interaction between KASH and SUN domains, GFP-KASH, SS–HA-Sun1–KDEL, or both proteins were translated in reticulocyte lysate containing [35S]methionine/cysteine in either the presence or absence of microsomes (Totals). Anti-GFP immunoprecipitation of a fraction of each sample revealed the pull-down of SS–HA-Sun1–KDEL by GFP-KASH when both proteins were cotranslated in the presence of microsomes (arrow). A slightly faster migrating band (asterisk) was detected in the absence of microsomes. However, this band originates in the GFP-KASH translation and is unrelated to HA-Sun1L–KDEL. Molecular masses are indicated in kD. (E) Immunoprecipitation of HeLa cell lysates with anti-Sun2 antibodies coprecipitates a very large anti-nesp2G immunoreactive protein (arrow). HC indicates the position of immunoglobulin heavy chains. These data suggest a significant interaction between KASH and Sun proteins that must involve their lumenal domains. These findings allow us to propose a model for the LINC complex (F) in which nuclear components, including lamins, bind to the INM SUN domain proteins. They, in turn, bind to the KASH domain of the actin-associated giant nesprins on the ONM. Thus, the LINC complex establishes a physical connection between the nucleoskeleton and the cytoskeleton.