Abstract
Fabry disease is an inborn error of glycosphingolipid catabolism resulting from a deficiency of lysosomal enzyme α-galactosidase A. The major clinical manifestations of the disease, such as stroke, cardiac dysfunction, and renal impairment, are thought to be caused by vasculopathy due to progressive accumulation of globotriaosylceramide in vascular endothelial cells. The pathogenesis of the vasculopathy has not been elucidated. Since in vitro studies using primary endothelial cells are hampered by the limited lifespan of these cells, the availability of cultured endothelial cells with an extended lifespan is critical for the study of the vasculopathy of Fabry disease. We therefore generated an endothelial cell line from a Fabry hemizygote by introduction of human telomerase reverse transcriptase gene. The cell line has markedly extended lifespan compared to parental primary cells. The cells stably express many key markers of endothelial cells such as von Willebrand factor, CD31, CD34, and endothelial nitric oxide synthase (eNOS) and retain functional characteristics such as uptake of acetylated low density lipoprotein, responsiveness to angiogenic growth factors, up-regulation of eNOS production upon extracellular stimuli, and formation of tube-like structures on Matrigel basement membrane matrix. The cells show significantly reduced activity of α-galactosidase A compared with primary endothelial cells from normal individuals and accumulate globotriaosylceramide in lysosomes. This cell line will provide a useful in vitro model of Fabry disease and will facilitate systematic studies to investigate pathogenic mechanisms and explore new therapeutic approaches for Fabry disease.
Keywords: Fabry disease, alpha-galactosidase A deficiency, lysosomal storage disorders, globotriaosylceramide, endothelial cells, hTERT
Introduction
Fabry disease is an X-linked metabolic disorder caused by an insufficient activity of α-galactosidase A (α-Gal A, EC 3.2.1.22) [1]. As a result of the enzymatic defect, glycosphingolipids with terminal α-D-galactosyl residues (predominantly globotriaosylceramide, Gb3) accumulate systemically, particularly in vascular endothelial and smooth-muscle cells [2]. Clinical manifestations include renal failure, myocardial infarction, and stroke. Swollen endothelial cells (ECs) due to lysosomal deposition of Gb3 were thought to play a role in ischemic vascular events. Recently, altered essential cerebral hyper-perfusion and endothelial-dependent vascular reactivity have been demonstrated in Fabry patients and in a mouse model with α-Gal A deficiency [3–5], suggesting that endothelial dysfunction might be associated with the vascular manifestations. However, the relationship between abnormal glycosphingolipid metabolism and the vascular disease remains unclear.
In vitro cultures of ECs are important models for studying the role of endothelium in many physiological/pathological conditions. To study the pathogenic mechanism of Fabry disease, cultured ECs from Fabry patients would be useful. Primary endothelial cultures obtained from patients and a mouse model with Fabry disease have been reported previously [6–8]. However, like other human somatic cells in culture, primary ECs enter replicative senescence after a limited number of divisions. The limited lifespan and significant alterations of gene expression and cell function occurring with senescence in vitro [9–13] limit the utility of primary human ECs for in vitro studies. Thus, an immortalized or life-extended endothelial cell line possessing distinct disease phenotype is desirable. Recently, several groups have reported the generation of immortalized human ECs by introduction of human telomerase reverse transcriptase (hTERT) gene [13–17]. These telomerase-expressing ECs have been shown not only to have extended lifespan, but also to retain many of the characteristics of primary ECs and normal karyotype, without a transformed phenotype.
In this study, we established microvascular endothelial cell line with an extended lifespan from a patient with Fabry disease by a retroviral transduction of the hTERT gene, and characterized the expression of both endothelial markers and a Fabry phenotype.
Materials and Methods
Cell cultures and gene transduction
Microvascular endothelial cells were obtained from a 3-mm punch forearm skin biopsy from Fabry patients and non-Fabry controls, using a modification of the method of Normand and Karasek [18]. All subjects who had skin biopsies gave their written informed consent on a study protocol approved by the institutional review board of the National Institute of Neurological Disorders and Stroke. Primary cultures were grown on gelatin-coated plates in MCDB 131 medium (Invitrogen, Carlsbad, CA) supplemented with 10 ng/ml recombinant human basic fibroblast growth factor (bFGF) (PeproTech, Rocky Hill, NJ), 100 μg/ml sodium heparin (Sigma, St. Louis, MO), 1.25 μM forskolin (Sigma), and 10% Cosmic Calf Serum (Hyclone, Logan, UT). At the first passage, ECs were positively selected using anti-IgG magnetic beads (Cortex Biochem, San Leandro, CA) coated with anti-human CD31 (PECAM-1) antibody (Sigma) before replating on fibronectin-coated dishes.
The retrovirus pBabe-hTERT was generously provided by Dr. Woody Wright (University of Texas Southwestern Medical Center, Dallas, TX). The vector expresses hTERT and puromycin resistance gene from Moloney murine leukemia virus long terminal repeat (LTR) and SV40 early promoter respectively. Primary endothelial cells at passage 2–4 were infected with this amphotropic retrovirus in the presence of 8 μg/ml polybrene, and transduced cells were selected with puromycin at 1 μg/ml for 7 days. Drug-resistant cells were pooled and expanded. Cells were maintained with EGM-2 medium (Clonetics, Cambrex, Walkersville, MD), supplemented with 2% fetal bovine serum (FBS), hydrocortisone, hEGF, VEGF, hFGF-B, R3-IGF-1, ascorbic acid, and heparin. The medium was changed every 1–2 days. When cultures were approximately 80% confluent, cells were trypsinized and subcultured at a split ratio of 1:3. Primary dermal microvascular endothelial cells from normal adult (HMVEC) were purchased from Clonetics and were maintained in EGM-2 medium.
Quantitative RT-PCR
Total RNA was extracted from the cells with RNeasy kit (Qiagen, Hilden, Germany) according the manufacturer’s instructions. After treatment with DNase I (Invitrogen), reverse transcription was performed using SuperScript II with 0.2 μg RNA and random hexamers (Invitrogen). Quantitative PCR was performed using an ABI prism 7900 sequence detection system with SYBR Green dye with primers specific for hTERT gene (amplicon: 95 bp) as described previously [19]. The absence of nonspecific amplification was confirmed by dissociation curve analysis. 18S rRNA was used as internal control and detected by TaqMan probe and primers (ABI). The absence of contamination of genomic DNA in the templates was confirmed by conventional RT-PCR using samples with or without reverse transcription.
Clonogenic analysis
Cells were trypsinized and seeded at low density (2 cells/cm2). Two hours after plating, single cells were marked under the microscope. Medium was changed daily and the number of colonies (approximately ≥2 mm diameter) produced from the marked single cells was calculated 12–13 days after initial plating.
Senescence-associated β-galactosidase staining
Cytochemical staining for senescence-associated β-galactosidase (SA β-gal) at pH 6.0 was performed according to the method of Dimiri et al. [20] and cells were briefly counterstained with Nuclear Fast Red.
Immunostaining
Cells were seeded on glass coverslips or plastic chambers. Cells were fixed with 2% paraformaldehyde (PFA) in PBS for 10 min at room temperature and treated with 0.3% Triton X-100 in PBS (0.3% PBST) for 5 min. After blocking with 10% normal goat serum (NGS) in 0.01% PBST for 1 h, cells were incubated with primary antibodies in 5% NGS/0.01% PBST overnight at 4°C. For detection of cell surface markers, permeablization by 0.3% Triton X-100 was omitted. Antibody binding was visualized by incubation with Alexa 594- or 488-conjugated goat anti-mouse or anti-rabbit IgG secondary antibodies (Invitrogen). The cells were counterstained with DAPI, mounted, and observed with a Zeiss Axioplan fluorescence microscope equipped with a Spot RT digital camera. The primary antibodies and concentrations used were: mouse monoclonal antibodies to CD31/PECAM-1 (Sigma; 1:100), CD34 (Dako, Glostrup, Denmark; 1:20), and Gb3 (Seikagaku, Tokyo, Japan; 1:300), and rabbit polyclonal antibody to Von Willebrand Factor (vWF, Abcam, Cambridge, MA; 1:300).
Western blot analysis
Cells were lysed by brief sonication in a buffer consisting of 50 mM Tris (pH 7.5), 150 mM NaCl, 1mM EDTA, 20 mM CHAPS and protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentration was determined with the BCA protein assay reagent (Pierce, Rockford, IL). Lysates were denatured with LDS sample buffer (Invitrogen) and heated at 70°C for 10 min. Approximately 20 to 25 μg of total proteins were separated in 3–8% Tris-Acetate NuPAGE gels (Invitrogen) and blotted to PVDF membranes. After blocking with 5% skim milk overnight at 4°C, the membranes were incubated with mouse monoclonal antibody to eNOS (Clone3, 1:800, Transduction Laboratories, San Jose, CA) in blocking buffer for 2 h at RT. The signal was detected by SuperSignal West Femto kit (Pierce). As loading control, actin was detected with a monoclonal antibody to β-actin (Sigma).
Uptake of DiI-labeled acetylated low-density lipoprotein (DiI-Ac-LDL)
Cells were incubated in growth medium containing 10 μg/ml of DiI-Ac-LDL (Biomedical Technologies Inc, Stoughton, MA) at 37°C for 4 h. After washing with PBS, cells were fixed with 2% PFA for 10 min at RT.
Proliferation assays
To measure responsiveness to growth factors, cells were seeded onto 96-well plates at a density of 104 cells/cm2. On the following day, medium was changed to human endothelial serum-free medium (SFM) (Gibco, Invitrogen) supplemented with 2% FBS and incubated for an additional 2 days. Under these conditions, the cells enter mitotic quiescence. Cultures were then incubated for 3 days with human endothelial SFM with 2% FBS containing various concentrations (0–50 ng/ml) of bFGF (Gibco, Invitrogen) or vascular endothelial growth factor (VEGF, PeproTech). At the end of treatment, cell numbers were determined by colorimetric MTT assay. Cells were incubated in a medium containing 0.5 mg/ml MTT (Sigma) at 37°C for 3 h. The formazan crystals were dissolved in DMSO and absorbance was measured at a wavelength of 570 nm, with a reference wavelength of 630 nm. Results were expressed as a ratio of the absorbance of the stimulated wells to the absorbance of the non-stimulated wells.
Matrigel tubulogenesis assay
The capacity for angiogenenic tube formation was assessed by culturing cells on Matrigel™ matrix using the BD BioCoat Angiogenesis System: Endothelial Cell Tube Formation, (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Briefly, Matrigel Matrix in pre-coated 96 well plates was thawed at 4°C for 7 hrs and was allowed to polymerize at 37°C for 30 min. Cells suspended in EGM-2 medium were seeded onto the matrix at a density of 2 × 104 cells/well and were incubated for 16 hrs at 37°C. Tubulogenesis was evaluated by phase contrast microscopy. Primary human dermal microvascular cells were used as a positive control.
Soft agar culture
A single-cell suspension (1 × 104 cells/ml) in EGM-2 growth medium containing 0.33% agar (3ml) was added to 6 cm dishes containing a 0.5% agar base layer (5 ml). The cells were cultured for 2 weeks and colony formation was assessed using a phase contrast microscope.
α-Galactosidase A enzyme assay
Activity of α-galactosidase A was determined by fluorimetric assay at pH 4.4 using 4-methylumbellferyl-α-D-galactopyrandoside as substrate, as previously described [21].
Results
Generation of endothelial cells with an extended lifespan from Fabry patients
Using magnetic beads coated with monoclonal antibody to CD31, we obtained high purity primary ECs from standard skin biopsies from several Fabry patients and non-Fabry controls. The endothelial origin was confirmed by their typical cobblestone morphology, uptake of DiI-Ac-LDL, and immunostaining for PECAM-1 (data not shown). There were no apparent differences in morphology or growth rate between the cells from patients and controls. These cells had a lifespan of approximately 5–7 passages in culture.
To obtain cell lines with an extended lifespan, ECs from 7 individuals, including Fabry patients and normal controls, were infected at passages 2–4 with a retrovirus encoding hTERT. After selection with puromycin, successfully transduced cells were pooled. During expansion, we obtained 3 pure endothelial cultures. The other 4 endothelial cultures were overgrown by contaminating fibroblasts and excluded from the experiment. Two out of 3 pure cultures (normal controls, aged 26 and 29) showed moderately extended lifespan compared to primary cells but eventually ceased growth with senescent morphology at passage 15 and 13 respectively. Only one culture (Fabry hemizygote with R112H mutation, donor age 64) exhibited a significantly extended lifespan and was expanded and characterized in this study.
Expression of transduced hTERT in the Fabry patient-derived ECs was confirmed by real time RT-PCR. In contrast to hTERT mRNA in primary parental cells, which was almost undetectable, the mRNA level in the transduced cells increased significantly and the level is much higher than 293 cells, a transformed human embryonic kidney cell line known to express detectable levels of hTERT (Fig 1A).
Fig 1. hTERT-induced lifespan extension of Fabry ECs.
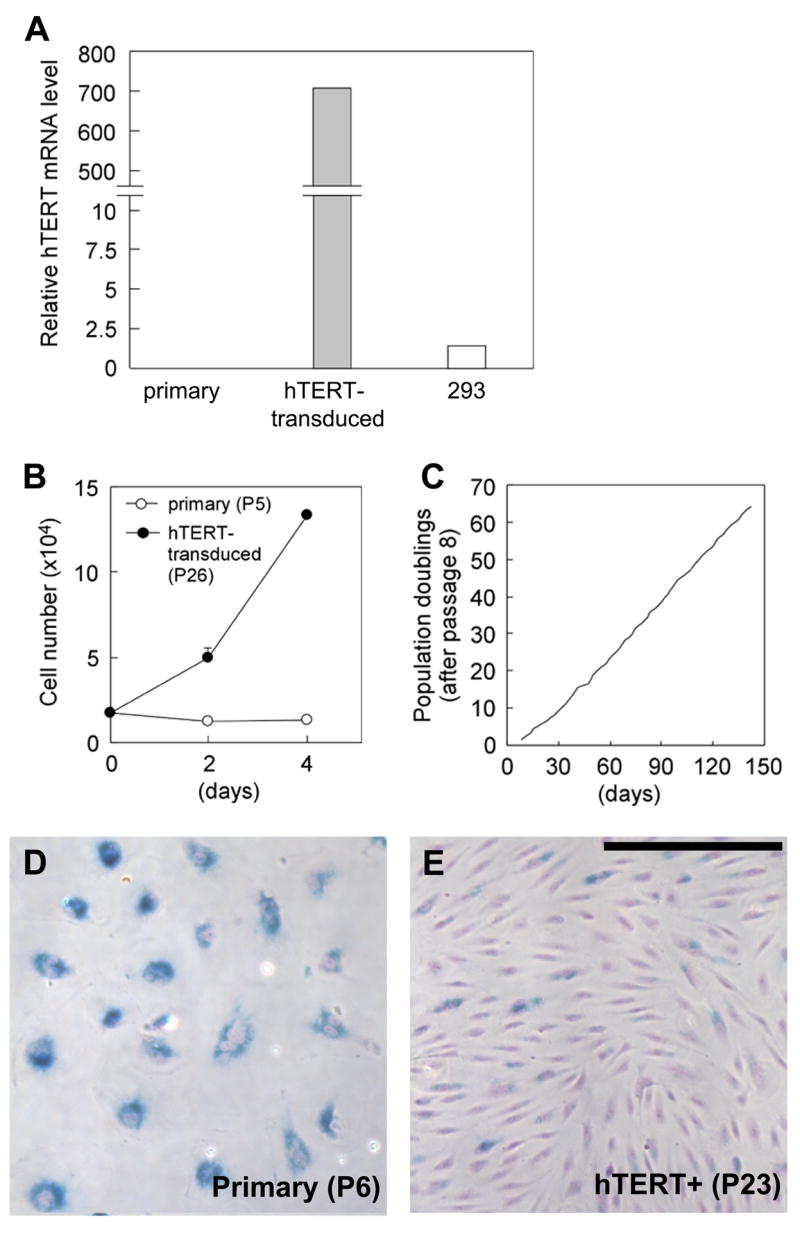
(A) Expression level of hTERT was assessed by quantitative RT-PCR. (B) Growth rates of primary parental cells at passage 5 and hTERT-transduced cells at passage 26. hTERT-transduced cells proliferate actively with doubling time of 33.6 h, while primary cells essentially did not divide. (C) Population doubling (counted from passage 8) of hTERT transduced cells. (D and E) Microscopic photographs of SA β-gal cytochemistry of primary parental cells at passage 6 (D) and hTERT-transduced cells at passage 23 (E). Photos were taken at the same magnification. Scale bar: 250 μm
Expression of hTERT markedly extended the lifespan of the cells compared to non-transduced parental cells. The parental primary cells enter replicative senescence at passage 5–6, with typical flattened and enlarged cell morphology (Fig 1B and D). By contrast, hTERT-transduced cells proliferated actively up to at least passage 45, and did not show senescence-related morphology (Fig 1E). The culture has been maintained continuously for more than 5 months, and the cells are continuously proliferating without a decrease in the rate of growth (Fig 1B and C).
β-Galactosidase (β-gal) activity detected by cytochemical assay at pH 6.0 is known as senescence-associated (SA) β-gal and is considered as a biomarker of replicative senescence of some human cells [20]. Most parental primary cells at passage 6 were strongly positive for SA β-gal activity (Fig 1D). At passage 23, only approximately 30% of hTERT-transduced cells were positive for SA β-gal staining and positive cells generally showed a much lower intensity than that of the primary cells (Fig 1E).
To determine the percentage of proliferating cells in hTERT-transduced cultures, colony-forming efficiency was evaluated at passage 19. Among single cells analyzed, 53.9% (41/76) cells formed readily isolatable colonies (≥2 mm diameter) within 12 days after plating and 30.3% (23/76) remained single cells at analysis. This single cell population reflects senescent cells when they were seeded and their proportion is consistent with the results of the SA β-gal staining described above. The remaining 15.8% (12/76) of cells formed small colonies with limited cell numbers. To obtain homogeneous proliferating populations, 10 single cell-derived clones were isolated and expanded. Similar to the mass cultures, all clones exhibited about 30% SA β-gal positive cells. The colony-forming efficiency in the one clone (#1B) analyzed was 48.6% (18/37).
Collectively, by ectopic expression of hTERT, senescence was overcome in this EC culture from a Fabry patient and the lifespan of the cells was extended to more than 8 times that of primary ECs. These cells could be considered as an established cell line and were designated as IMFE1 (Immortalized Fabry Endothelial cell line-1).
Endothelial characteristics of the IMFE1 cell line
During the initial stage of expansion, IMFE1 cells showed essentially similar morphology to parental cells with round or polygonal cell bodies and cobblestone-like appearance when confluent. With increasing passage number, however, the proportion of cells with elongated cell bodies and spindle-shaped phase-dark perinuclear regions gradually increased. The cells with this morphology became predominant around passage 11–14. These cells form a moderately swirling monolayer at confluence (Fig 2A).
Fig 2. Characterization of IMFE1 cell line.
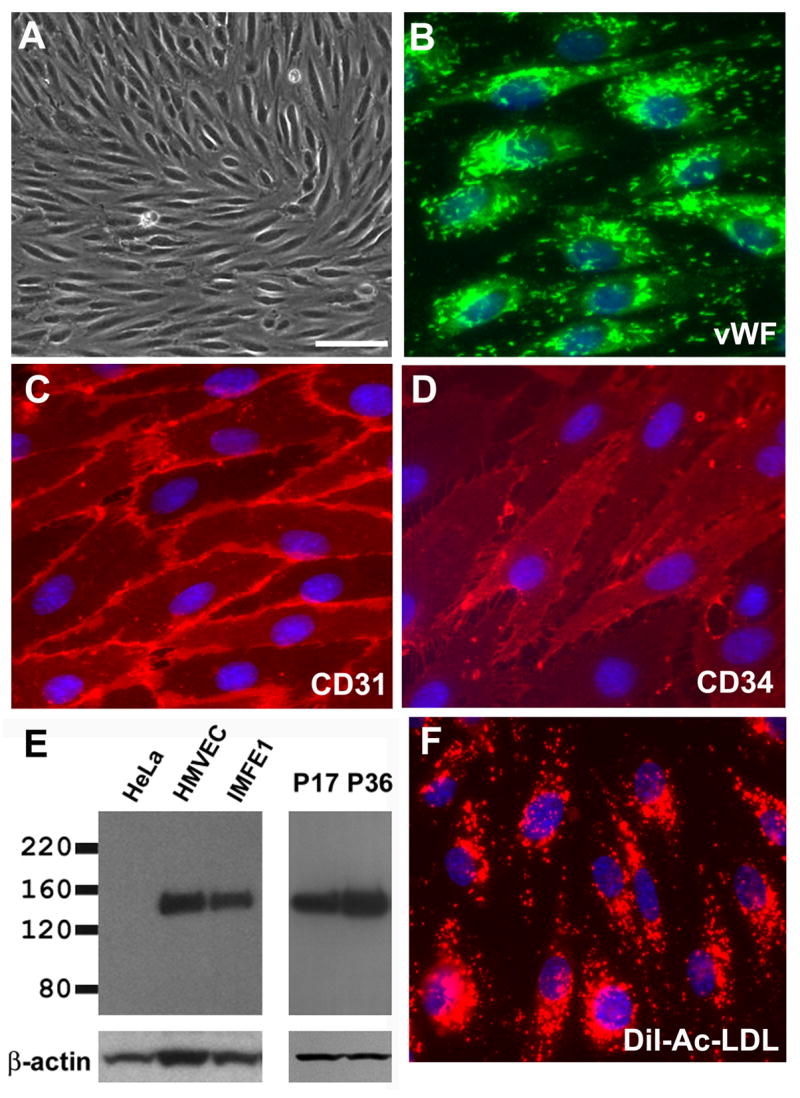
(A) Morphology of IMFE1 cells under phase contrast microscope. (B–D) Immunofluorescence staining for vWF (B), CD31 (C) and CD34 (D). (E) eNOS expression analyzed by Western blot analysis. Left panel: Identification of eNOS expression in IMFE1 cell line. Primary human microvascular endothelial cells (HMVEC) and HeLa cells were used as positive and negative control respectively. Right panel: Persistent eNOS expression in IMFE1 cells at earlier and later passages (P). β-actin was used as loading control. (F) Uptake of DiI-Ac-LDL. Incorporated DiI (red) shows characteristic lysosomal-staining. Scale bar in (A): 100 μm
IMFE1 cells expressed characteristic markers of ECs. Almost all cells express von Willebrand factor (vWF), which is considered the most reliable marker for ECs. vWF-immunoreactive signals showed rod-shaped structures characteristic of Weibel-Palade bodies [22] throughout the cytoplasm (Fig 2B). All cells expressed PECAM-1/CD31, a highly specific endothelial marker, with intense signal at intercellular junctions (Fig 2C). Most of the cells were also positive for CD34. Staining showed a diffuse surface pattern with increased intensity on microvilli, similar to that reported in early passages of primary ECs [12] (Fig 2D). IMFE1 cells also expressed another endothelial marker, eNOS, with protein levels similar to primary HMVEC (Fig 2E). In addition, all IMFE1 cells took up DiI-Ac-LDL (Fig 2F) indicating the presence of scavenger receptors for acetylated LDL. The expression of these markers was examined approximately every 5 passages during expansion and no obvious changes in expression pattern or level were detected up to passage 45. One of the important functions of ECs is the responsiveness to angiogenic growth factors. We evaluated the mitogenic response of IMFE1 cells to bFGF and VEGF. Both growth factors stimulated the proliferation of the cells significantly (Fig 3A). Basic FGF stimulated the proliferation of IMFE1 cells in a dose-dependent manner over the concentration range 0.5–50 ng/ml. VEGF induced the maximum response of the cells at 5 ng/ml. In primary ECs, VEGF and bFGF, in addition to other extracellular stimuli, regulate the expression of eNOS [23–26]. Treatment with VEGF or bFGF for 48 h induced up-regulation of eNOS expression in IMFE1 cells (Fig 3B). Similar to the proliferative response, the effect of bFGF on the induction of eNOS expression was dose-dependent over a wide range of concentrations (2, 10 and 100 ng/ml). VEGF at concentrations of 2–10 ng/ml also had a minor effect on eNOS expression. At 100 ng/ml, however, there was a significant induction of eNOS expression. Another important feature of endothelial cells is their capacity to form capillary-like structures under appropriate conditions in vitro. The tube-forming ability was evaluated by exposing IMFE1 cells (passage 17) to Matrigel. IMFE1 cells formed typical capillary tube-like structures and the pattern of tubule network is indistinguishable to that formed by primary HMVEC at passage 6 (Fig 3C).
Fig 3. Responsiveness of IMFE1 cells to angiogenic growth factors and growth on Matrigel.
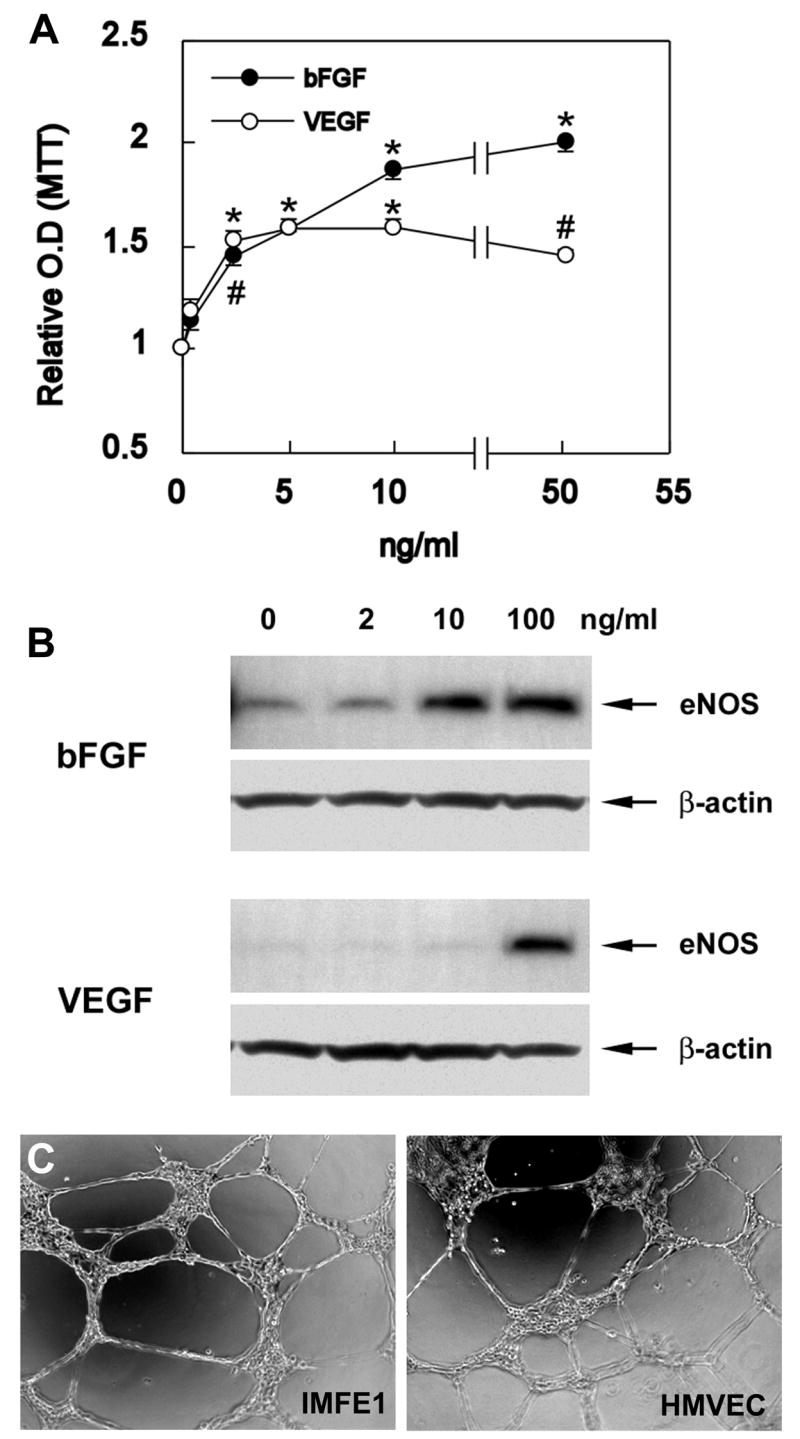
(A) Growth response curves of IMFE1 cells 72hs after stimulation with bFGF and VEGF. Results are expressed as a ratio of the absorbance of the stimulated wells to the absorbance of the non-stimulated wells. Data are presented as mean ± SE (n = 6). (*P<0.0003, #P<0.002, stimulated wells compared with non-stimulated wells, two-tailed Student’s t test) (B) eNOS expression analyzed by Western blot. IMFE1 cells were incubated with medium containing 0–100 ng/ml VEGF or bFGF for 48 hr. β-Actin was used as loading control. (C) Tube formation assay. IMFE1 cells grown on Matrigel extracellular matrix formed tube-like structures that were similar to those formed by primary HMVEC.
In addition, IMFE1 cells maintained anchorage-dependence for growth, since they did not form colonies in soft agar, suggesting they are not transformed. In the same experiment ECV304, a spontaneously transformed cell line formed large colonies (>100 cells) within 2 weeks (data not shown).
Fabry phenotype of IMFE1 cells
The R112H mutation in α-Gal A gene was confirmed by DNA sequencing in IMFE1 cells, indicating the culture was of patient origin and not cross-contaminated by other cells. The activity of α-Gal A in the homogenate from IMFE1 cells was significantly lower than that of non-Fabry primary ECs (16% of average activity of 4 controls) (Fig 4A). In immunostaining against Gb3, many IMFE1 cells showed punctuate lysosomal-distribution of positive signal (Fig 4B). The staining showed marked heterogeneity in signal intensity among cells. Roughly 15–30% cells exhibited intense, large positive spots with the others being weakly positive or negative. No significant change in staining pattern was shown during continuous passage. Treatment of IMFE1 cells with 0.4 IU/ml recombinant α-Gal A enzyme for 48 h significantly reduced lysosomal accumulation of Gb3 detected by immunostaining (Fig 4B, inset). This further confirmed the elevated level of Gb3 in the cells was due to deficient α-Gal A activity. No significant positive signal to Gb3 could be seen in the normal control, HMVEC (data not shown).
Fig 4. Fabry phenotype of IMFE1 cell line.
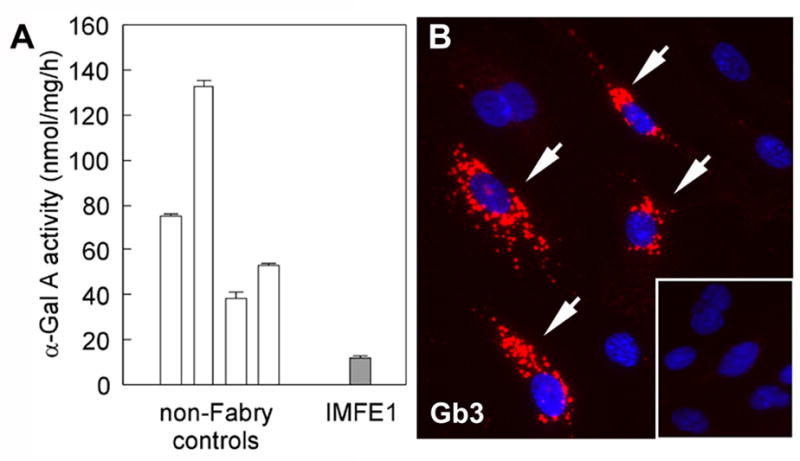
(A) α-Gal A activity of IMFE1 cells and primary ECs from four non-Fabry controls. The data are presented as mean ± SE (n ≥ 3). (B) Immunostaining of Gb3 in IMFE1 cells. Lysosomal-distribution of positive signal is apparent in some of the cells (arrows). Inset: Immunostaining of the cells after treatment with recombinant α-Gal A (0.4 IU/ml) for 48 hr.
Discussion
In the present study, we successfully established a dermal microvascular endothelial cell line (IMFE1) with an extended lifespan from a patient with Fabry disease. Senescence was overcome in this cell line by ectopic over-expression of hTERT. The cells proliferate actively and constantly with an increased lifespan of more than 8-fold over that of primary cells. About 50% of the cells are clonogenic. However, only 1 out of 3 transductants acquired significant extension of lifespan, suggesting that telomerase-mediated immortalization is individual donor-dependent or requires additional genetic events. This efficiency of cell line generation is similar to observations in a previous report on generation of bone marrow- and peripheral blood-derived ECs [15]. Other studies suggest that a combination of SV40 large-T antigen and hTERT can lead to more effective immortalization [27, 28]. Whether additional ectopic oncogene expression is required for consistent telomerase-induced cellular immortalization remains unclear.
Ectopic expression of telomerase not only extended the lifespan of IMFE1 cells but also possibly contributed to the preservation of their specific phenotype and functions. It has been shown that the expression of eNOS and CD34 decreases with cell doubling level in primary ECs [11,12] and such a decrement could be prevented by hTERT [11]. Thus, stable expression of endothelial markers in IMFE1 cells over time may be due to, at least in part, the stable expression of telomerase. Most IMFE1 cells are positive for CD34, confirming the cells are of blood microvascular origin. It has been shown that CD34 is selectively expressed in blood vessels but not lymphatic microvasculature in the human skin [29,30].
The expression of eNOS is under complex and tight regulation [31] and can be induced by several extracellular stimuli in primary ECs [23–26]. In addition to a detectable basal expression, eNOS expression in IMFE1 cells could be increased by VEGF or bFGF in a dose-dependent fashion. This finding suggests that at least some of the signaling pathways regulating eNOS expression are preserved in IMFE1 cells. This property might be particularly important for the utility of IMFE1 cells in the investigation of the pathogenesis of Fabry disease, since the eNOS/NO pathway plays a critical role in the development of vascular disorders, including atherosclerosis [32], and it has been suggested that dysregulation of NO pathway occurs in Fabry disease [33].
IMFE1 cells showed a decreased α-Gal A activity and accumulation of Gb3, the principal biochemical abnormalities of Fabry disease. Gb3 content in IMFE1 cells varied markedly among cells. This pattern was similar in both pooled cultures and single cell-derived clones and therefore was probably not due to a heterogeneous genetic background. Significant deposition of Gb3 in endothelium in Fabry patients is thought to be due to endocytosis of circulating Gb3 [2,6] and it is possible that the low level of serum in the culture (2%) allowed some cells to scavenge more Gb3 from the medium than others. In addition, glycolipid content of cultured cells has been shown to vary with mitogenic status [34, 35] and senescence [36], which could also produce heterogeneous staining in non-synchronized cultures.
The pathology of Fabry disease is characterized by accumulation of lipid deposits in the vascular endothelium and smooth muscle cells. The mechanism by which this accumulation causes symptoms of disease is still unknown. Historically, it has been thought that accumulation of lipid in the endothelium of capillaries and other small vessels resulted in infarction and ischemia leading to tissue damage [2]. However, more recent studies have indicated that endothelial dysfunction plays a significant role in the pathenogenesis of this disease. Altarescu and co-workers demonstrated significant blunting of endothelial response to acetylcholine stimulation resulting in significant changes in blood flow in Fabry patients [3]. In addition, DeGraba et al. have demonstrated an increase in markers of endothelial activation in plasma of patients with this disease, resulting in a proinflammatory state [37].
Microvessel ECs are of particular interest in the pathology of Fabry disease. One of the diagnostic hallmarks of this disease is the presence of angiokeratomas which consist of tangles of dilated and thrombic small vessels containing lipid-laden endothelium [2] indicating that microvessels of the skin are one of the primary sites of disease involvement. Global expression profiling of endothelial cells from large and small vessels from different anatomic sites has indicated that, in addition to the general expression of genes specific to all cells of endothelial origin, microvessel ECs differentially express genes involved in interactions with circulating blood cells and responses to pathogens [38]. These interactions contribute to inflammatory reactions. Of particular interest, Chi and co-workers found that microvascular endothelial cells express receptors for a variety of paracrine signals from neuroglial cells, suggesting close interaction between microvessel ECs and peripheral nerves. Nearly all Fabry patients suffer from neuropathic pain in their hands and feet which can debilitating. Closer investigation of the interaction of microvessel ECs with neuronal cell types may be useful in understanding the origin of this pain.
We have shown in this study that the IMFE1 cell line is an authentic in vitro model of Fabry disease. It possesses both endothelial characteristics and the Fabry phenotype. The extended replicative capacity and stable cellular phenotype of this cell line will facilitate systematic studies to investigate pathogenic mechanisms and explore new therapeutic approaches for Fabry disease.
Acknowledgments
We would like to thank Dr. Woody Wright (University of Texas Southwestern Medical Center, Dallas, TX) for kindly providing the retrovirus pBabe-hTERT. This work was funded by the Intramural Program of the National Institute of Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramide trihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 2.Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. McGraw-Hill; New York: 2001. pp. 3733–3774. [Google Scholar]
- 3.Altarescu G, Moore DF, Pursley R, Campia U, Goldstein S, Bryant M, Panza JA, Schiffmann R. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke. 2001;32:1559–1562. doi: 10.1161/01.str.32.7.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007 doi: 10.1016/j.jns.2007.01.053. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Heare T, Alp NJ, Priestman DA, Kulkarni AB, Qasba P, Butters TD, Dwek RA, Clarke K, Channon KM, Platt FM. Severe endothelial dysfunction in the aorta of a mouse model of Fabry disease; partial prevention by N-butyldeoxynojirimycin treatment. J Inherit Metab Dis. 2007;30:79–87. doi: 10.1007/s10545-006-0473-y. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DL, Desnick RJ. Molecular pathology of Fabry’s disease. Physical and kinetic properties of alpha-galactosidase A in cultured human endothelial cells. Biochim Biophys Acta. 1978;538:195–204. doi: 10.1016/0304-4165(78)90346-x. [DOI] [PubMed] [Google Scholar]
- 7.Hasholt L, Sorensen SA. Lysosomal alpha-galactosidase in endothelial cell cultures established from a Fabry hemizygous and normal umbilical veins. Hum Genet. 1986;72:72–76. doi: 10.1007/BF00278821. [DOI] [PubMed] [Google Scholar]
- 8.Shu L, Murphy HS, Cooling L, Shayman JA. An in vitro model of Fabry disease. J Am Soc Nephrol. 2005;16:2636–2645. doi: 10.1681/ASN.2005040383. [DOI] [PubMed] [Google Scholar]
- 9.Pagani F, Zagato L, Maier JA, Ragnotti G, Coviello DA, Vergani C. Expression and alternative splicing of fibronectin mRNA in human diploid endothelial cells during aging in vitro. Biochim Biophys Acta. 1993;1173:172–178. doi: 10.1016/0167-4781(93)90178-g. [DOI] [PubMed] [Google Scholar]
- 10.Maier JA, Statuto M, Ragnotti G. Senescence stimulates U937-endothelial cell interactions. Exp Cell Res. 1993;208:270–274. doi: 10.1006/excr.1993.1246. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ Res. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 12.Delia D, Lampugnani MG, Resnati M, Dejana E, Aiello A, Fontanella E, Soligo D, Pierotti MA, Greaves MF. CD34 expression is regulated reciprocally with adhesion molecules in vascular endothelial cells in vitro. Blood. 1993;81:1001–1008. [PubMed] [Google Scholar]
- 13.Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, Bronstein A, Chiu CP, Herron GS. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 14.Venetsanakos E, Mirza A, Fanton C, Romanov SR, Tlsty T, McMahon M. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp Cell Res. 2002;273:21–33. doi: 10.1006/excr.2001.5424. [DOI] [PubMed] [Google Scholar]
- 15.MacKenzie KL, Franco S, Naiyer AJ, May C, Sadelain M, Rafii S, Moore MA. Multiple stages of malignant transformation of human endothelial cells modeled by co-expression of telomerase reverse transcriptase, SV40 T antigen and oncogenic N-ras. Oncogene. 2002;21:4200–4211. doi: 10.1038/sj.onc.1205425. [DOI] [PubMed] [Google Scholar]
- 16.Nisato RE, Harrison JA, Buser R, Orci L, Rinsch C, Montesano R, Dupraz P, Pepper MS. Generation and characterization of telomerase-transfected human lymphatic endothelial cells with an extended life span. Am J Pathol. 2004;165:11–24. doi: 10.1016/S0002-9440(10)63271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman DA, Folkman J. Maintenance of G1 checkpoint controls in telomerase-immortalized endothelial cells. Cell Cycle. 2004;3:811–816. [PubMed] [Google Scholar]
- 18.Normand J, Karasek MA. A method for the isolation and serial propagation of keratinocytes, endothelial cells, and fibroblasts from a single punch biopsy of human skin. In Vitro Cell Dev Biol Anim. 1995;31:447–455. doi: 10.1007/BF02634257. [DOI] [PubMed] [Google Scholar]
- 19.Bieche I, Nogues C, Paradis V, Olivi M, Bedossa P, Lidereau R, Vidaud M. Quantitation of hTERT gene expression in sporadic breast tumors with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000;6:452–459. [PubMed] [Google Scholar]
- 20.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusiak JW, Quirk JM, Brady RO. Purification and properties of the two major isozymes of α-Galactosidase A from human placenta. J Biol Chem. 1978;253:184–190. [PubMed] [Google Scholar]
- 22.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol. 1995;269:H550–555. doi: 10.1152/ajpheart.1995.269.2.H550. [DOI] [PubMed] [Google Scholar]
- 24.Arnet UA, McMillan A, Dinerman JL, Ballermann B, Lowenstein CJ. Regulation of endothelial nitric-oxide synthase during hypoxia. J Biol Chem. 1996;271:15069–15073. doi: 10.1074/jbc.271.25.15069. [DOI] [PubMed] [Google Scholar]
- 25.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 26.Kostyk SK, Kourembanas S, Wheeler EL, Medeiros D, McQuillan LP, D’Amore PA, Braunhut SJ. Basic fibroblast growth factor increases nitric oxide synthase production in bovine endothelial cells. Am J Physiol. 1995;269:H1583–H1589. doi: 10.1152/ajpheart.1995.269.5.H1583. [DOI] [PubMed] [Google Scholar]
- 27.O’Hare MJ, Bond J, Clarke C, Takeuchi Y, Atherton AJ, Berry C, Moody J, Silver AR, Davies DC, Alsop AE, Neville AM, Jat PS. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci U S A. 2001;98:646–651. doi: 10.1073/pnas.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krump-Konvalinkova V, Bittinger F, Unger RE, Peters K, Lehr HA, Kirkpatrick CJ. Generation of human pulmonary microvascular endothelial cell lines. Lab Invest. 2001;81:1717–1727. doi: 10.1038/labinvest.3780385. [DOI] [PubMed] [Google Scholar]
- 29.Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001;280:F193–206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease-Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation. 2001;104:1506–1512. doi: 10.1161/hc3801.096352. [DOI] [PubMed] [Google Scholar]
- 34.Vukelic Z, Kalanj-Bognar S. Cell density-dependent changes of glycosphingolipid biosynthesis in cultured human skin fibroblasts. Glycoconj J. 2001;18:429–437. doi: 10.1023/a:1016066816457. [DOI] [PubMed] [Google Scholar]
- 35.Kanda T, Ariga T, Kubodera H, Jin HL, Owada K, Kasama T, Yamawaki M, Mizusawa H. Glycosphingolipid composition of primary cultured human brain microvascular endothelial cells. J Neurosci Res. 2004;78:141–150. doi: 10.1002/jnr.20228. [DOI] [PubMed] [Google Scholar]
- 36.Venable ME, Webb-Froehlich LM, Sloan EF, Thomley JE. Shift in sphingolipid metabolism leads to an accumulation of ceramide in senescence. Mech Ageing Dev. 2006;127:473–480. doi: 10.1016/j.mad.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 37.DeGraba T, Azhar S, Dignat-George F, Brown E, Boutière B, Altarescu G, McCarron R, Schiffmann R. Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000;47:229–233. [PubMed] [Google Scholar]
- 38.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]


