Abstract
The operative mortality rate of liver resection has decreased from 10% to 20% before the 1980s to <5% in most specialized hepatobiliary centers nowadays. The most important factor for better outcome is reduced blood loss due to improvement in surgical techniques. Liver transection is the most challenging part of liver resection, associated with a risk of massive hemorrhage. Understanding the segmental anatomy of the liver and delineation of the proper transection plane using intraoperative ultrasound are prerequisites to safe liver transection. Clamp crushing and ultrasonic dissection are the two most widely used transection techniques. In recent years, new instruments using different types of energy for coagulation or sealing of vessels have been developed for liver transection. These include radiofrequency devices, Harmonic Scalpel, Ligasure and TissueLink dissecting sealer. Whether these new instruments, used alone or in combination with clamp crushing or ultrasonic dissection, improve the safety of liver transection has not been clearly demonstrated. The use of the vascular stapler for transection of major intrahepatic vascular trunks is also gaining popularity. These new instruments are particularly useful in liver transection during laparoscopic liver resection. Adjunctive measures such as intermittent Pringle maneuver and low central venous pressure anesthesia are also useful measures to reduce the risk of hemorrhage. This article reviews the safety and efficacy of different techniques of liver transection, with particular attention to evidence from randomized controlled trials available in the literature.
Introduction
Hepatic resection is a surgical procedure of great challenge because of the risk of massive bleeding during liver transection and the complicated biliary and vascular anatomy in the liver. The history of development of surgical techniques of liver resection is largely a struggle against hemorrhage from liver transection. Before the 1980s, hepatic resection was associated with a mortality rate of 10–20%, and a common cause of operative mortality was hemorrhage 1,2,3. Nowadays, the hospital mortality rate of liver resection is 5% or lower in most specialized centers 4,5,6,7,8. While better patient selection in terms of liver function reserve is an important factor 9, reduction of blood loss and perioperative transfusion is another major factor for improved perioperative outcome 7,8. Excessive hemorrhage and perioperative blood transfusion not only increase the risk of operative morbidity and mortality, but also jeopardize long-term survival after resection of liver malignancies because the associated immunosuppression leads to a higher risk of tumor recurrence 7,10. Recent reduction in perioperative blood transfusion after resection of hepatocellular carcinoma has contributed to improved long-term patient survival 11.
Finger fracture or clamp crushing has been a standard technique for transection of liver parenchyma. Over the past 20 years, technological advances have led to the development of specific instruments for liver transection, such as the ultrasonic dissector, water jet, Harmonic Scalpel, Ligasure, and TissueLink dissecting sealer. Other advances in operative techniques have also contributed to reduction in blood loss during liver transection. These include better delineation of the transection plane with the use of intraoperative ultrasound, and better inflow and outflow control. Inflow occlusion and low central venous pressure anesthesia have been widely used to reduce bleeding from inflow vessels and hepatic veins in the transection surface. This article reviews the current techniques of liver transection and evidence from the literature on the efficacy of different techniques.
Delineation of the proper transection plane
The delineation of a proper transection plane is important not only for adequate tumor-free margin in resection of liver tumors, but also to avoid inadvertent injuries to major intrahepatic vessels or bile duct pedicles. The delineation of the transection plane starts with critical appraisal of the preoperative contrast computed tomography (CT) scan to define the relationship between a tumor and the major intrahepatic vessels or bile duct pedicles, which can be further evaluated in the operation by intraoperative ultrasound (IOUS) (Figure 1). In patients with a large right or left lobe tumor, IOUS allows evaluation of the relationship between the tumor and the middle hepatic vein, and hence helps the surgeon to decide whether extended hepatic resection is needed for tumor clearance. Similarly, information on the relationship between the tumor and the right or left hepatic vein or portal pedicle may influence the decision on the type of resection and the transection plane. Without the knowledge of the relationship between the tumor and the major intrahepatic structures, unexpected damage to such structures can occur during transection, leading to massive bleeding or bile duct injuries, and sometimes tumor exposure at the transection plane. In general, a tumor-free margin of 1 cm is considered necessary for curative purpose, although the exact significance of tumor margin in hepatic resection for liver cancers, especially hepatocellular carcinoma, remains controversial 12. In cirrhotic patients with borderline liver function reserve, preservation of liver parenchyma may take priority over a wide resection margin.
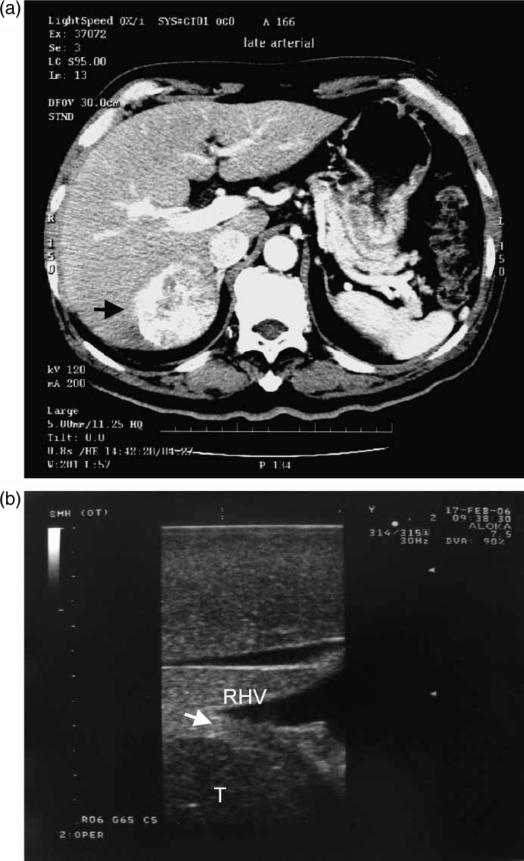
Figure 1. .
A hepatocellular carcinoma planned for right posterior sectionectomy (arrow, A). Intraoperative ultrasound showed the tumor (T) abutting the right hepatic vein (RHV) (arrow, B). A right posterior sectionectomy including the right hepatic vein was performed to avoid tumor exposure.
IOUS plays a particularly important role in segmental resection of the liver. Better understanding of the segmental anatomy of the liver has led to a wider practice of segmental resection, which sacrifices less liver parenchyma compared with a formal right or left hepatectomy, while it improves the chance of tumor clearance compared with a non-anatomical wedge resection 13. IOUS allows localization of the segmental portal pedicle, and some surgeons use dye injection into the segmental portal vein to stain the segment and more clearly delineate the transection plane before resection 14. Clamping of the vascular pedicles to demonstrate the ischemic demarcation and intrahepatic glissonian access to the biliovascular pedicle are other techniques useful in delineation of the transection plane for segmental resection 15,16.
Techniques of liver transection
Finger fracture/clamp crushing
Control of bleeding during transection of the liver is a major challenge for liver surgeons. Hepatic transection is particularly difficult in cirrhotic liver due to the fibrotic nature of liver tissue. The presence of bleeding tendency in cirrhotic patients also increases the risk of massive bleeding. The finger fracture technique, which involves crushing of liver parenchyma by fingers under inflow occlusion to isolate vessels and bile ducts for ligation, was first introduced by Lin et al. in 1958 17. This technique was subsequently improved through the use of surgical instruments such as a small Kelly clamp for blunt dissection (clamp crushing) 18. Nowadays the clamp crushing technique is still one of the most widely used techniques of liver transection 5,6,19.
Ultrasonic dissection
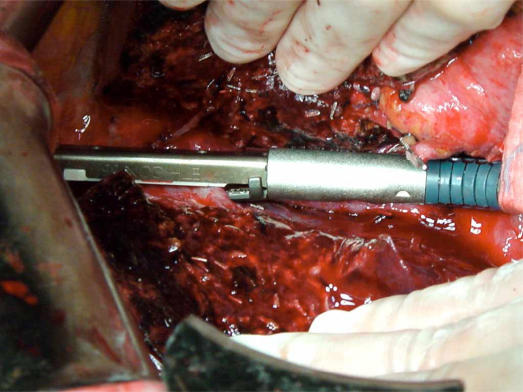
In many centers, including the author's center, ultrasonic dissection using the Cavitron Ultrasonic Surgical Aspirator (CUSA, Tyco Healthcare, Mansfield, MA, USA) has become the standard technique of liver transection (Figure 2). With this technology, the liver parenchyma tissue is fragmented with ultrasonic energy and aspirated, thus exposing vascular and ductal structures that can be ligated or clipped with titanium hemoclips. A previous retrospective study from the author's center showed that the ultrasonic dissector resulted in lower blood loss, lower morbidity, and lower mortality compared with the clamp crushing technique 20. Furthermore, ultrasonic dissection resulted in a wider tumor-free margin because of a more precise transection plane. However, in a randomized controlled trial comparing clamp crushing and ultrasonic dissection conducted by a Japanese group, no significant differences in blood loss, transection speed, tumor exposure at the surgical margin, or postoperative morbidity were observed 21. Using a grading system to grade the quality of liver transection, the group demonstrated better quality of hepatectomy using the clamp crushing method. However, it is noteworthy that the group is among one of the best in terms of experience with clamp crushing technique, which had been their transection technique all along before the randomized trial. The result with each transection technique is significantly affected by the individual surgeon's experience with the respective technique.
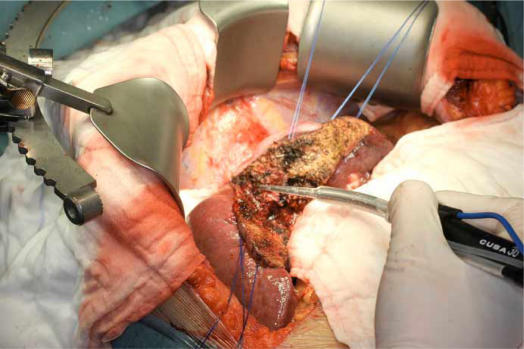
Figure 2. .
Ultrasonic dissection of liver parenchyma using Cavitron ultrasonic surgical aspirator (CUSA).
Water jet
The water jet dissector employs a pressurized jet of water instead of ultrasonic energy to fragment the liver parenchyma tissue and expose the vascular and ductal structures. In the only available prospective randomized trial of water jet in the literature, in which 31 patients underwent liver resection using water jet and another 30 patients underwent liver resection using CUSA, water jet transection reduced blood loss, blood transfusion, and transection time compared with CUSA 22. However, this technique has not become as popular as CUSA. One disadvantage of both CUSA and water jet in liver transection is the long transection time because of the need for ligation or clipping of individual vessels. There are also concerns of increased risk of venous air embolism with either CUSA or water jet technique, although this appears to be a clinically rare problem 23,24. Both CUSA and water jet techniques are quite good for dissecting out major hepatic veins when tumors are in proximity. This allows for delineation of hepatic veins, particularly at the junction with the inferior vena cava, and prevents positive margin.
Harmonic Scalpel
More recently, new technologies that allow sealing of small vessels during transection of liver parenchyma have been developed, with the aim of reducing blood loss and transection time. These technologies can be used alone for transection of the liver parenchyma, or in combination with clamp crushing or CUSA. One of these technologies is ultrasonic shear (Harmonic Scalpel, Ethicon Endo-Surgery, Cincinnati, OH, USA), which uses ultrasonically activated shears to seal small vessels between the vibrating blades 25. The blade's longitudinal vibration with a frequency of 55.5 kHz can dissect liver parenchyma easily. The coagulation effect is caused by protein denaturation, which occurs as a result of destruction of the hydrogen bonds in proteins and generation of heat in the vibrating tissue Blood vessels up to 2–3 mm in diameter are coagulated on contact with the vibrating blade. The tissue-cutting effect derives from a saw mechanism in the direction of the vibrating blade.
This instrument has been used for liver transection in both open and laparoscopic liver resections, with no biliary leakage reported in a study of 41 patients 26. In contrast, a recent non-randomized study that compared Harmonic Scalpel with the conventional clamp crushing method showed that the use of Harmonic Scalpel was associated with a significantly increased rate of postoperative bile leakage, raising the concern that Harmonic Scalpel may not be effective in sealing bile ducts 27. The instrument may also have a limitation in dissecting the liver parenchyma around the main trunk of hepatic veins, since it is difficult to achieve sufficient control of bleeding from large vessels using the Harmonic Scalpel alone 28. However, when combined with the use of ultrasonic dissection, Harmonic Scalpel may reduce blood loss 29. This remains to be proven by a randomized trial, which is not available in the literature yet.
While the benefit of the use of Harmonic Scalpel in open liver resection remains uncertain, it is commonly used in laparoscopic liver resection, especially for resection of peripheral lesions, because of the difficulty in using CUSA or water jet in the laparoscopic setting 30. The Harmonic Scalpel may also be useful in transection of cirrhotic liver, for which the clamp crushing technique may not be very effective.
Ligasure
Ligasure (Valley Lab, Tyco Healthcare, Boulder, CO, USA) is another device designed to seal small vessels using a different principle. By a combination of compression pressure and bipolar radiofrequency (RF) energy, it causes shrinkage of collagen and elastin in the vessel wall, and it is effective in sealing small vessels up to 7 mm in diameter (Figure 3). Preliminary studies on the use of this device in liver transection have demonstrated its effectiveness in sealing intrahepatic vessels 31,32. Similar to the Harmonic Scalpel, there is also some concern as to its capability to maintain seal integrity in larger bile ducts based on an in vivo animal study 33. In a recent study of the use of Ligasure for liver transection in 30 patients, there was no clinical evidence of bile leakage 34. In that study, the instrument was effective in liver transection in normal or near-normal liver, but it failed to achieve hemostasis in three patients with cirrhotic liver. A recently published randomized controlled trial demonstrated that the use of Ligasure in combination with a clamp crushing technique resulted in lower blood loss and faster transection speed in minor liver resections compared with the conventional technique of electric cautery or ligature for controlling vessels in the transection plane 35. In that study, the bile leakage rate with the use of Ligasure was 9% compared with 3% in the conventional technique group, but the difference was not statistically significant. The efficacy of Ligasure in sealing bile duct needs to be investigated with further randomized trials. Similar to the Harmonic Scalpel, laparoscopic Ligasure is a useful instrument for liver transection in the setting of laparoscopic resection of peripheral liver lesions 36.
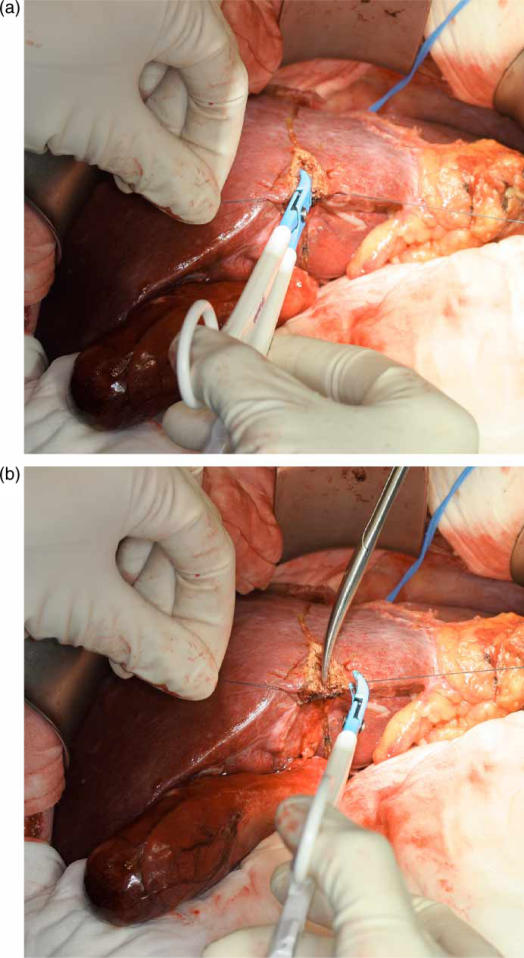
Figure 3. .
Use of Ligasure for liver transection (A). The sealed vessel can be divided without clipping or ligation (B).
TissueLink dissecting sealer
A new technology using saline-linked RF energy (TissueLink Medical, Inc., Dover, NH, USA) has been developed for liver transection. In this instrument, saline runs to the tip of the electrode to couple RF energy to the liver surface and achieve coagulation. In a recent study, combined use of a Floating Ball coagulator that uses this principle together with Ligasure has been shown to reduce blood loss compared with the conventional technique of control of intrahepatic vessels during clamp crushing transection of the liver 32. The author has recently reported a preliminary experience on the use a TissueLink dissecting sealer (TissueLink DS3.5C dissecting sealer, TissueLink Medical, Inc.) for liver transection without the aid of any other devices such as CUSA 37. This device has a pointed tip that allows transection and sealing of vessels simultaneously (Figure 4). In the reported preliminary experience of 10 cases, including 2 cases of right hepatectomy, the median blood loss was 100 ml (range 30–700 ml) and no postoperative bile leakage was observed. This instrument may also be used in laparoscopic liver resection.
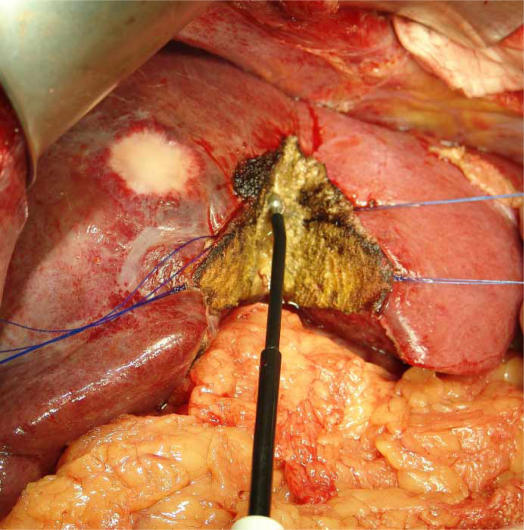
Figure 4. .
Use of TissueLink dissecting sealer for liver transection. The liver is coagulated and transected simultaneously by the pointed dissecting tip.
Radiofrequency-assisted liver transection
RF ablation (RFA) is a relatively new modality of treatment for liver tumors 38. A new technique of liver transection using RF thermocoagulation has been described recently 39. In this technique, a Cool-tip® RF electrode (Radionics Inc., Burlington, MA, USA) is inserted along the transection plane serially 1–2 cm apart, and RF energy is applied for 1–2 min to create overlapping cylinders of coagulated tissue, followed by transection of the coagulated liver using a simple scalpel. In a preliminary study of 15 cases of mainly segmental or wedge resection reported by Weber et al. 39, the mean blood loss was only 30±10 ml, and no complications such as bile leakage were observed. Another group also reported low blood loss using this technique in liver resection 40.
The technique has the advantage of simplicity compared with the aforementioned transection techniques. One potential disadvantage of this technique is the sacrifice of parenchymal tissue in the liver remnant, with a 1 cm wide necrotic tissue at the transection margin, which may be critical in cirrhotic patients who require major liver resection. However, the additional 1 cm tissue necrosis along the transection margin increases the actual resection margin, which may be an advantage in some cases of liver resection for cancers. There is a concern about the possible thermal injury to the hilar structures and hepatic veins when using this technique for major liver resection, although right hepatectomy using this technique has been reported 41. Thus far, this technique has not been compared with conventional techniques of liver transection in any randomized study. The author has used this technique in laparoscopic wedge liver resection and found it a convenient and safe method with minimal blood loss in this setting. Recently, an in-line RFA device consisting of multiple parallel RFA electrodes that can be deployed to varying depths in the hepatic parenchyma has been specifically developed for liver transection. This device has been shown to reduce bleeding compared with CUSA in a pilot study 42. The role of this device in hepatic resection remains to be evaluated in future studies.
Comparison of different liver transection techniques
The choice of transection techniques is currently a matter of preference of surgeons, as there are few data from prospective randomized trials that compared different techniques. It has been shown in small prospective randomized trials that clamp crushing or water jet may be preferable to CUSA in terms of quality of transection or speed of transection 21,22. However, the results of these trials remain to be validated by larger-scale trials. CUSA dissection is still a widely used technique worldwide. Recently, a randomized trial compared four methods of liver transection, namely clamp crushing, CUSA, Hydrojet, and dissecting sealer, with 25 patients in each group 43. In that study, clamp crushing was associated with the fastest transection speed, lowest blood loss, and lowest blood transfusion requirement. Furthermore, clamp crushing was the most cost-effective technique. However, in that study, clamp crushing was performed with the Pringle maneuver, whereas the other techniques were performed without the Pringle maneuver. This might have resulted in bias in favor of clamp crushing. Further prospective randomized studies are needed to determine which transection technique is the best. In the comparison of different techniques, apart from the efficacy in transection with low blood loss, the relative speed of transection and the potential complications are other parameters to be considered. Furthermore, the use of special instruments for transection is costly, especially when two instruments are used in combination for transection and hemostasis. It is difficult to compare the relative cost of different transection instruments because some are reusable whereas others are designed for single use, and the cost of the same instrument varies substantially in different countries. Nonetheless, the cost of these various techniques should play a part in the surgeon's decision as to whether to use them or not.
Use of vascular staples in liver transection
Staplers can be used in liver surgery for control of inflow and outflow vessels, or to divide liver parenchyma 44,45. It is particularly useful in dividing the major trunk of hepatic veins or the middle hepatic vein deep in the transection plane (Figure 5). Using the conventional technique of applying vascular clamp followed by suturing, slipping of the clamp or inadequate suture can result in massive blood loss that is difficult to control. Vascular staplers also can be used to divide the hepatic duct pedicle in right or left hepatectomy. The author prefers to divide the hepatic duct pedicle during hepatic transection instead of dividing the duct extrahepatically to avoid leaving an ischemic stump of hepatic duct, which may increase the risk of biliary fistula. The use of a vascular stapler to divide the hepatic duct saves time from suturing. However, caution has to be taken not to narrow the hepatic duct confluence, especially during a right or left extended hepatectomy for a large tumor encroaching on the liver hilum, which leaves little room for application of the stapler 46. The use of a stapler for transection of the liver parenchyma may be applicable in minor wedge resection or left lateral segmentectomy when the liver tissue is not too bulky. One problem associated with the use of a stapler for liver transection is increased risk of bile leak, since the stapler is not very effective in sealing small bile ducts.
Figure 5. .
Use of a vascular stapler for transection of middle hepatic vein in the transection plane during extended right hepatectomy.
Inflow and outflow vascular control
Inflow occlusion by clamping of the portal triad (Pringle maneuver) is frequently used to reduce bleeding during hepatic transection. A previous prospective randomized trial conducted in the author's center demonstrated that the use of intermittent Pringle maneuver for 20 min with a 5 min clamp-free interval during liver transection reduced blood loss 47. However, there is a limit to the duration that the Pringle maneuver can be applied. Prolonged application of the Pringle maneuver for a total of >120 min may have deleterious effects on liver function 48. Belghiti et al. 49 demonstrated in a prospective randomized trial that liver parenchyma tolerance was better with intermittent Pringle maneuver than with continuous Pringle maneuver, especially in patients with chronic liver disease. In a recent randomized trial, however, it was shown that no significant difference was observed in blood loss of liver resection with or without inflow occlusion 50. In the author's center, the Pringle maneuver was used less frequently in liver transection for the past few years with increased experience of the surgical team, while low blood loss with a transfusion rate of <5% could still be achieved 8.
Other authors have used total hepatic vascular exclusion instead of the Pringle maneuver to reduce blood loss in major liver resection. Conflicting data were reported in two randomized trials published thus far. One randomized trial demonstrated that hepatic vascular exclusion was associated with unpredictable hemodynamic intolerance and increased postoperative complications compared with the Pringle maneuver 51. However, another randomized trial showed that selective hepatic vascular exclusion was well tolerated by patients and was associated with less intraoperative blood loss, better postoperative liver function, and shorter hospital stay compared with the Pringle maneuver 52. In the author's center, total vascular exclusion was never used 8. Other authors also have reported that hepatic resection, even for tumors close to the hepato-caval junction, could be performed without total vascular exclusion 53.
Low central venous pressure
A major source of bleeding during liver transection is hepatic vein branches in the deeper part of the transection plane. Bleeding from openings made in major trunks of hepatic veins cannot be controlled even with new high technology devices such as the argon beam coagulator and TissueLink dissecting sealer. Such bleeding can be aggravated in the presence of a high central venous pressure. Furthermore, a high central venous pressure makes repair of injury to the hepatic vein or inferior vena cava difficult.
One of the most important advances in liver resection in recent years is the practice of low central venous pressure anesthesia, achieved by a combination of posture change, fluid restriction, diuretics, vasodilators, and anesthetic agents such as Isoflurane that produce vasodilatation. The central venous pressure should be lowered to <5 mmHg, provided that the hemodynamic status is stable. The patient is placed in a 15° Trendelenburg position to counteract the iatrogenic hypovolemia and help protect the kidney. One concern as regards low central venous pressure is the increased possibility of air embolism. However, clinically significant air embolism is seldom observed, and the benefit of reduced bleeding with low central venous pressure outweighs the risk of air embolism. Several studies have demonstrated that low central venous pressure anesthesia is well tolerated by most patients and is effective in reducing blood loss, with results superior to that of total vascular exclusion 54,55. A recent randomized controlled trial involving 50 patients with either low central venous pressure 2–4 mmHg or normal central venous pressure showed that the use of low central venous pressure during liver transection led to significantly reduced blood loss and length of hospital stay 56.
Conclusions
Improvement in the techniques of liver transection is one of the most important factors for improved safety of hepatectomy in recent years. The use of intraoperative ultrasound aids delineation of the proper transection plane. Clamp crushing and ultrasonic dissection are currently the two most popular techniques of liver transection. The role of new instruments such as ultrasonic shear and RFA devices in liver transection remains unclear, with few data available in the literature. The role of the Pringle maneuver seems to be decreasing with improved transection technique. However, it remains a useful technique in reducing bleeding from inflow vessels, especially for surgeons with less experience in liver resection. Maintenance of low central venous pressure remains an important adjunctive measure to reduce blood loss in liver transection.
As clear data for comparison of various liver transection techniques are lacking, currently the choice of technique is often based on the individual surgeon's preference. However, certain general recommendations can be made based on existing data and the author's experience. Clamp crushing is a low-cost technique but it requires substantial experience to be used effectively for liver transection, especially in the cirrhotic liver. CUSA can be used in both cirrhotic and non-cirrhotic liver, and it is currently the standard liver transection technique in the author's center. It is associated with low blood loss and it has a well-established safety record, with low risk of bile leak. It is particularly useful in major hepatic resections when dissection of the major branches of the hepatic veins is required, or in cases where the tumor is in close proximity to a major hepatic vein, as it allows clear dissection of the hepatic vein from the tumor. The main disadvantage of the CUSA technique is slow transection. Newer instruments such as the Harmonic Scalpel, Ligasure and TissueLink Dissector enhance the capability of hemostasis and allow faster transection. However, they lack the preciseness of CUSA in dissection of major hepatic veins, and they may be associated with increased risk of bile leak. When used alone, they are more suited for wedge or segmental resection in which dissection of the major hepatic veins is not required, and they are particularly useful in laparoscopic liver resection. They can also be used in combination with CUSA for sealing of vessels, but this increases the cost substantially. RFA-assisted transection is probably the most speedy liver transection technique. However, the risk of thermal injury to major bile duct is a serious concern and its use is probably restricted to minor resection. It is imperative that further randomized trials be performed to compare different liver transection techniques, not only to compare their efficacy in reducing blood loss and safety, but also to evaluate their relative cost-effectiveness, which is a major issue in the current era of expanding cost of medical care as a result of rapid advances in medical technologies.
References
- 1.Fortner JG, Blumgart LH. A historic perspective of liver surgery for tumors at the end of the millennium. J Am Coll Surg. 2001;193:210–22. doi: 10.1016/s1072-7515(01)00910-3. [DOI] [PubMed] [Google Scholar]
- 2.Thompson HH, Tompkins RK, Longmire WP., Jr Major hepatic resection. A 25-year experience. Ann Surg. 1983;197:375–88. doi: 10.1097/00000658-198304000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740–8. [PubMed] [Google Scholar]
- 4.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvenet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Gonen M, Fong Y, De Matteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 7.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–9. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10(2 Suppl 1):S39–S45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 10.Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–30. doi: 10.1001/archsurg.134.10.1124. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–51. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billingsley KG, Jarnagin WR, Fong Y, Blumgart LH. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg. 1998;187:471–81. doi: 10.1016/s1072-7515(98)00231-2. [DOI] [PubMed] [Google Scholar]
- 14.Takayama T, Makuuchi M. Intraoperative ultrasonography and other techniques for segmental resections. Surg Oncol Clin N Am. 1996;5:261–9. [PubMed] [Google Scholar]
- 15.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–80. doi: 10.1308/003588402760978148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado MA, Herman P, Figueira ER, Bachella T, Machado MC. Intrahepatic Glissonian access for segmental liver resection in cirrhotic patients. Am J Surg. 2006;192:388–92. doi: 10.1016/j.amjsurg.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Lin TY, Tsu K, Mien C, Chen C. Study on lobectomy of the liver. J Formosa Med Assoc. 1958;57:742–9. [Google Scholar]
- 18.Lin TY. A simplified technique for hepatic resection: the crush method. Ann Surg. 1974;180:285–90. doi: 10.1097/00000658-197409000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun HC, Qin LX, Lu L, Wang L, Ye QH, Ren N, et al. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg. 2006;93:422–66. doi: 10.1002/bjs.5260. [DOI] [PubMed] [Google Scholar]
- 20.Fan ST, Lai EC, Lo CM, Chu KM, Liu CL, Wong J. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117–20. doi: 10.1002/bjs.1800830138. [DOI] [PubMed] [Google Scholar]
- 21.Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922–8. doi: 10.1001/archsurg.136.8.922. [DOI] [PubMed] [Google Scholar]
- 22.Rau HG, Wichmann MW, Schinkel S, Buttler E, Pickelmann S, Schauer R, et al. Surgical techniques in hepatic resections: Ultrasonic aspirator versus Jet-Cutter. A prospective randomized clinical trial. Zentralbl Chir. 2001;126:586–90. doi: 10.1055/s-2001-16573. [DOI] [PubMed] [Google Scholar]
- 23.Smith JA. Possible venous air embolism with a new water jet dissector. Br J Anaesth. 1993;70:466–7. doi: 10.1093/bja/70.4.466. [DOI] [PubMed] [Google Scholar]
- 24.Koo BN, Kil HK, Choi JS, Kim JY, Chun DH, Hong YW. Hepatic resection by the Cavitron Ultrasonic Surgical Aspirator increases the incidence and severity of venous air embolism. Anesth Analg. 2005;101:966–970. doi: 10.1213/01.ane.0000169295.08054.fa. [DOI] [PubMed] [Google Scholar]
- 25.Gertsch P, Pelloni A, Guerra A, Krpo A. Initial experience with the harmonic scalpel in liver surgery. Hepatogastroenterology. 2000;47:763–6. [PubMed] [Google Scholar]
- 26.Schmidbauer S, Hallfeldt KK, Sitzmann G, Kantelhardt T, Trupka A. Experience with ultrasound scissors and blades (UltraCision) in open and laparoscopic liver resection. Ann Surg. 2002;235:27–30. doi: 10.1097/00000658-200201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Ahmad SA, Lowy AM, Buell JF, Pennington LJ, Soldano DA, et al. Increased biliary fistulas after liver resection with the harmonic scalpel. Am Surg. 2003;69:815–19. [PubMed] [Google Scholar]
- 28.Sugo H, Mikami Y, Matsumoto F, Tsumura H, Watanabe Y, Kojima K, et al. Hepatic resection using the harmonic scalpel. Surg Today. 2000;30:959–62. doi: 10.1007/s005950070055. [DOI] [PubMed] [Google Scholar]
- 29.Aldrighetti L, Pulitano C, Arru M, Catena M, Finazzi R, Ferla G. “Technological” approach versus clamp crushing technique for hepatic parenchymal transection: a comparative study. J Gastrointest Surg. 2006;10:974–9. doi: 10.1016/j.gassur.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–61. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horgan PG. A novel technique for parenchymal division during hepatectomy. Am J Surg. 2001;181:236–7. doi: 10.1016/s0002-9610(01)00556-6. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto Y, Yamamoto J, Kokudo N, Seki M, Kosuge T, Yamaguchi T, et al. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166–72. doi: 10.1007/s00268-003-7167-5. [DOI] [PubMed] [Google Scholar]
- 33.Matthews BD, Pratt BL, Backus CL, Kercher KW, Mostafa G, Lentzner A, et al. Effectiveness of the ultrasonic coagulating shears, LigaSure vessel sealer, and surgical clip application in biliary surgery: a comparative analysis. Am Surg. 2001;67:901–6. [PubMed] [Google Scholar]
- 34.Romano F, Franciosi C, Caprotti R, Uggeri F, Uggeri F. Hepatic surgery using the Ligasure vessel sealing system. World J Surg. 2005;29:110–12. doi: 10.1007/s00268-004-7541-y. [DOI] [PubMed] [Google Scholar]
- 35.Saiura A, Yamamoto J, Koga R, Sakamoto Y, Kokudo N, Seki M, et al. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. Am J Surg. 2006;192:41–5. doi: 10.1016/j.amjsurg.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Constant DL, Slakey DP, Campeau RJ, Dunne JB. Laparoscopic nonanatomic hepatic resection employing the Ligasure device. JSLS. 2005;9:35–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Poon RT, Fan ST, Wong J. Liver resection using a saline-linked radiofrequency dissecting sealer for transection of the liver. J Am Coll Surg. 2005;200:308–13. doi: 10.1016/j.jamcollsurg.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, et al. Learning curve for radiofrequency ablation for liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–9. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–3. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stella M, Percivale A, Pasqualini M, Profeti A, Gandolfo N, Serafini G, et al. Radiofrequency-assisted liver resection. J Gastrointest Surg. 2003;7:797–801. doi: 10.1016/s1091-255x(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 41.Navarra G, Spalding D, Zacharoulis D, Nicholls JP, Kirby S, Costa I, et al. Bloodless hepatectomy technique. HPB. 2002;4:95–7. doi: 10.1080/136518202760378470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haghighi KS, Wang F, King J, Daniel S, Morris DL. In-line radiofrequency ablation to minimize blood loss in hepatic parenchymal transection. Am J Surg. 2005;190:43–7. doi: 10.1016/j.amjsurg.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Lersutel M, Selzner M, Petrowsky S, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–22. doi: 10.1097/01.sla.0000189121.35617.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fong Y, Blumgart LH. Useful stapling techniques in liver surgery. J Am Coll Surg. 1997;185:93–100. doi: 10.1016/s1072-7515(01)00889-4. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko H, Otsuka Y, Takagi S, Tsuchiya M, Tamura A, Shiba T. Hepatic resection using stapling devices. Am J Surg. 2004;187:280–4. doi: 10.1016/j.amjsurg.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Wang WX, Fan ST. Use of the Endo-GIA vascular stapler for hepatic resection. Asian J Surg. 2003;26:193–6. doi: 10.1016/S1015-9584(09)60301-8. [DOI] [PubMed] [Google Scholar]
- 47.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–13. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Yu WC, et al. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999;134:533–9. doi: 10.1001/archsurg.134.5.533. [DOI] [PubMed] [Google Scholar]
- 49.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvenet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–75. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capussotti L, Muratore A, Ferroro A, Massucco P, Ribero D, Polastri R. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br J Surg. 2006;93:685–9. doi: 10.1002/bjs.5301. [DOI] [PubMed] [Google Scholar]
- 51.Belghiti J, Noun R, Zante E, Ballet T, Sauvenet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155–61. doi: 10.1097/00000658-199608000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smyrniotis VE, Kostopanagiotou GG, Contis JC, Farantos CI, Voros DC, Kannos DC, et al. Selective hepatic vascular exclusion versus Pringle maneuver in major liver resections: prospective study. World J Surg. 2003;27:765–9. doi: 10.1007/s00268-003-6978-8. [DOI] [PubMed] [Google Scholar]
- 53.Torzilli G, Makuuchi M, Midorikawa Y, Sano K, Inoue K, Takayama T, et al. Liver resection without total vascular exclusion: hazardous or beneficial? An analysis of our experience. Ann Surg. 2001;233:167–75. doi: 10.1097/00000658-200102000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–6. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 55.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–5. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 56.Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935–9. doi: 10.3748/wjg.v12.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]