Figure 4.
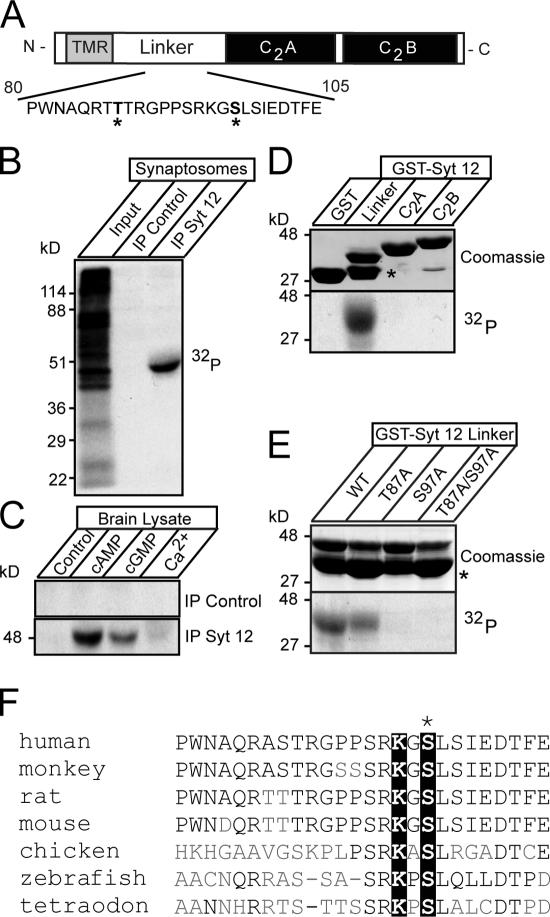
Synaptotagmin-12 is phosphorylated in a cAMP-dependent manner at serine97. (A) Schematic diagram of synaptotagmin-12 domain structure. A fragment of linker sequence containing two putative sites for PKA-dependent phosphorylation (T87 and S97, marked by asterisks) is illustrated below. (B) In vivo phosphorylation of native synaptotagmin-12. The proteins from rat brain synaptosomes preincubated with [32P]orthophosphate were extracted in detergent and immunoprecipitated with antibody to synaptotagmin-12 or preimmune serum. Incorporation of 32P was measured with x-ray film. Note that a single ∼50-kD band is observed in synaptotagmin-12, but not in control immunoprecipitation. (C) In vitro phosphorylation of native synaptotagmin-12. Proteins extracted from brain were immunoprecipitated with synaptotagmin-12 antibody or preimmune serum attached to Sepharose beads. The beads were incubated with brain cytosol mixed with 1 mM ATP and 10 μCi γ-[32P]ATP alone or with addition of 0.1 mM cAMP, 0.1 mM cGMP, or 1 mM Ca2+. Note that synaptotagmin-12 is strongly phosphorylated in a cAMP-dependent manner. (D and E) Identification of a functional PKA phosphorylation site in synaptotagmin-12 sequence. (D) Indicated fragments of synaptotagmin-12 were expressed as recombinant GST fusion proteins (top shows Coomassie staining of all fusion proteins; the asterisk corresponds to GST) and phosphorylated in vitro in the presence of catalytic subunit of PKA and γ-[32P]ATP. Note that only linker region of synaptotagmin-12 is phosphorylated. (E) Phosphorylation of wild-type synaptotagmin-12 linker GST fusion protein and linkers containing single alanine substitutions in residues T87, S97, or a combination of these mutations. Note that phosphorylation is completely abolished in S97A mutant. The asterisk corresponds to GST. (F) Multiple sequence alignment of synaptotagmin-12 homologues from different species shows the fragments of the linker sequences containing conserved PKA phosphorylation site (marked by black boxes).