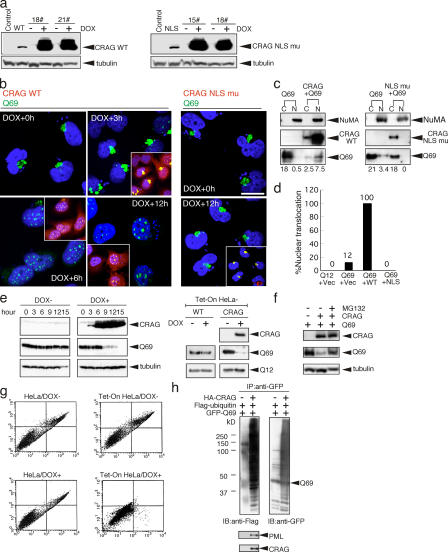
Figure 4.
CRAG promotes nuclear translocation and degradation of polyQ through the ubiquitin–proteasome pathway. (a) Establishment of DOX-inducible Tet-On HeLa cell lines expressing HA-CRAG WT and NLS mutants. Two clones each were treated with or without 1 μM DOX for 24 h and immunoblotted with anti-HA or anti-tubulin antibodies. Cells transiently transfected with control vector, CRAG WT, or NLS mutants were indicated in the first two lanes. (b) NLS-dependent nuclear translocation of Q69. At 24 h after transient transfection of Tet-On HeLa cells with Q69-myc (DOX + 0 h), cells were treated with DOX for the indicated times and immunostained with anti-myc (green), anti-HA antibodies (red), and Hoechst 33258 (blue). Merged images are shown in insets. (c) Biochemical analysis of nuclear translocation of Q69 by CRAG. Cytosolic (C) and nuclear (N) fractions from HeLa cells transfected with the indicated vectors were separated and immunoblotted with anti-HA, anti-myc, and anti–nuclear mitotic apparatus protein (NuMA; nuclear marker) antibodies. The optical density of Q69 bands (bottom) was analyzed by the NIH Image software. (d) Effect of CRAG WT and NLS mutants on the nuclear translocation of Q69. The percentage of cells showing nuclear translocation of Q69 was calculated from 100 HeLa cells transfected with the indicated vectors. (e) CRAG-dependent disappearance of Q69. HA-CRAG–inducible Tet-On HeLa cells were transfected with Q69-myc. At 24 h after transient transfection, cells were treated with or without 1 μM DOX for the indicated times and immunoblotted with anti-HA, anti-myc, and anti-tubulin antibodies. DOX alone did not affect the Q69 protein level in Tet-On HeLa cells without CRAG (WT; right). Note that CRAG expression did not affect the Q12 protein level. (f) MG132 blocked the CRAG-induced Q69 degradation. COS-7 cells expressing Q69-myc or Q69-myc/HA-CRAG were treated with or without 10 μM MG132 for 6 h and immunoblotted with anti-HA and anti-myc antibodies. (g) CRAG suppressed Q69-induced cell toxicity. Apoptosis in Tet-On HeLa cells without (left) or with (right) CRAG in the presence or absence of DOX was determined by FACS analysis using annexin-V/propidium iodide staining (right). Apoptotic cells were collected in the top right box. (h) GFP-CRAG enhanced in vivo ubiquitination of Q69. GFP-Q69 was immunoprecipitated from cells coexpressing GFP-Q69 and Flag-ubiquitin with or without GFP-CRAG and analyzed by immunoblot with the indicated antibodies. Bars, 20 μm.