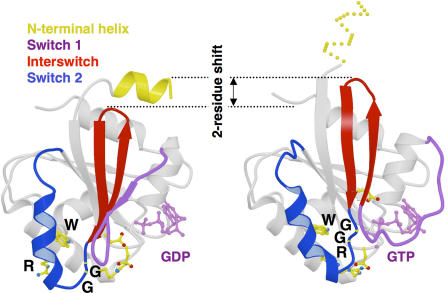
Figure 1.
The structural “air de famille.” In Arfs, Arls, and SAR proteins, the interswitch toggles from an unusual retracted conformation in the GDP-bound form that is fastened by the NH2-terminal helix to an exposed conformation in the GTP-bound form that is stabilized by the W/GG/R signature (shown here for ARF6-GDP and ARF6-GTP). This large conformational change, which involves a two-residue β-strand register shift in the core of the G domain, allows the nucleotide-binding site to detect remote interactions taking place at the NH2 terminus (reproduced from Pasqualato et al., 2001 with permission).