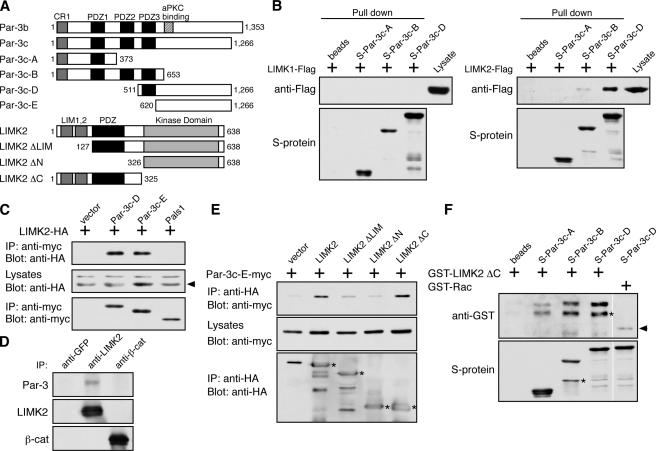
Figure 3.
Par-3 interacts with LIMK2 in vivo and in vitro. (A) Schematic diagrams of Par-3, LIMK2, and their various deletion fragments. Amino acid residue numbers are shown. Par-3c is a splice variant that lacks the aPKC-binding site. (B) Association of LIMK2 but not LIMK1 with recombinant Par-3 fragments. S-tagged Par-3 fragments immobilized on beads were incubated with COS cell lysates expressing Flag-tagged LIMK1 or LIMK2. Bound proteins were analyzed by Western blotting. (C) The COOH terminus of Par-3 is sufficient to associate with LIMK2. COS cells transfected with the indicated constructs were lysed, and the myc-tagged Par-3 fragments and Pals1 were immunoprecipitated by an anti-myc antibody. The associated LIMK2 was detected with an anti-HA antibody. Arrowhead points to LIMK2-HA. IP, immunoprecipitation. (D) Association of endogenous Par-3 and LIMK2 in MDCK cells. Endogenous LIMK2 was immunoprecipitated with an anti-LIMK2 antibody. Control immunoprecipitations were performed with anti-GFP and anti–β-catenin antibodies. (E) The NH2-terminal region of LIMK2 is required for association with the Par-3 COOH terminus. COS cells were transiently transfected with constructs expressing the COOH terminus of Par-3 (Par-3c-E) together with various LIMK2 deletion fragments. LIMK2 fragments were immunoprecipitated with an anti-HA antibody, and the associated Par-3c-E fragment was detected with an anti-myc antibody. Asterisks indicate LIMK2 fragments. (F) Binding of LIMK2 NH2 terminus to Par-3 fragments in vitro. GST–LIMK2 ΔC or GST-Rac was incubated with S-tagged Par-3 fragments immobilized on beads, and their interactions were analyzed by Western blotting. Asterisks indicate breakdown products. Arrowhead points to nonspecifically associated GST-Rac. White lines indicate that intervening lanes have been spliced out.