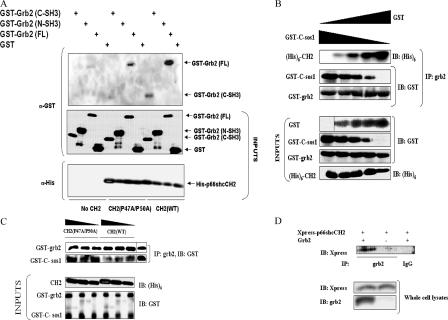
Figure 4.
P66shc and sos1 compete for binding to grb2. (A) Comparison of in vitro binding of the wild-type CH2 domain of p66shc (CH2[WT]) or the double proline mutant of the CH2 domain (CH2[P47A/P50A]) to GST-tagged full-length grb2 (FL) or the C- or N-SH3 domains of grb2. (B) In vitro dis-ruption of grb2–sos1 binding by CH2(WT) but not by CH2(P47A/P50A). Increasing amounts (250, 500, and 1,000 ng) of CH2 constructs were added to a preformed complex of GST-tagged full-length grb2 (GST-grb2) and GST–C-sos1. (C) In vitro binding assay showing displacement of CH2 from grb2 by C-sos1. Increasing amounts (50, 100, 200, and 400 ng) of GST–C-sos1 were added to preformed complex of GST-tagged full-length grb2 (GST-grb2) and hexahistidine-tagged CH2 ([His]6-CH2). GST was used to equalize the protein amount. (D, top) Coimmunoprecipitation of the CH2 domain and grb2 in COS7 cells overexpressing p66shcCH2 and grb2. The lysate from grb2 and p66shcCH2 overexpressing cells was also immunoprecipitated with control IgG and immunoblotted with Xpress antibody (right lane). (bottom) Expression of grb2 and p66shcCH2 in whole cell lysates. Black lines indicate that intervening lanes have been spliced.