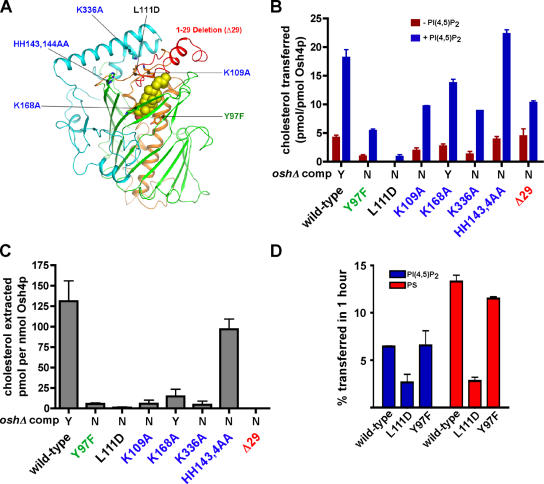
Figure 4.
Effect of mutations in Osh4p on cholesterol transport and extraction. (A) Location of the mutations shown in a stick model of Osh4p. The green residue is involved in ligand binding, the black residue is in the hydrophobic core, the blue residues are basic conserved residues, and the deletion of the NH2-terminal lid domain is red. A space-filling model of the cholesterol ligand is shown. (B) Cholesterol transport by wild-type and mutant Osh4p was measured. Donor and acceptor vesicles (1:10) were prepared as in Fig. 3 C, except that donor liposomes were prepared either with (blue bars) or without (red bars) 0.5% PI(4,5)P2. Vesicles were incubated at 30°C for 1 h, together with 40 pmol of Osh4p. The amount of transfer in control reactions lacking protein was subtracted to give the total amount of cholesterol transferred. (C) The amount of 14C-cholesterol extracted from liposomes (DOPC/14C-cholesterol, 99:1 mol%) by 200 pmol of wild-type or mutant Osh4p was determined as in Fig. 3 A. (D) Donor vesicles containing DOPC/egg PE/lactosyl-PE/3H-PI (60:20:10:1 mol%) or DOPC/egg PE/lactosyl-PE/3H-PS (60:20:10:10 mol%) were mixed with acceptor vesicles at a molar ratio of 1:10 and 40 pmol of wild-type or mutant Osh4p. Reactions were incubated for 1 h at 30°C. The amount of radiolabeled lipid transferred to acceptor vesicles minus the amount transferred in control reactions at 4°C was calculated. In B and C, the ability of the indicated mutants to complement oshΔ osh4-1 cells is shown (Y, growth; N, no growth; Im et al., 2005). In all graphs, values are the mean of three determinations. Error bars indicate the SEM. n = 3.