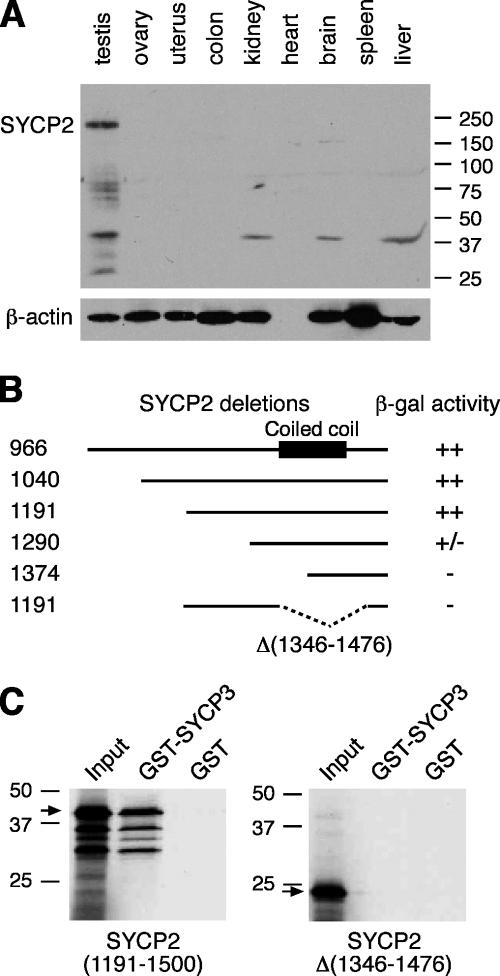
Figure 1.
Characterization of SYCP2 expression and its interaction with SYCP3. (A) Western blot analysis of SYCP2 in adult mouse tissues. 30 μg of protein extracts were loaded. β-actin served as a loading control. Rabbit anti-SYCP2 polyclonal antibodies were used (1:500). (B) Identification of SYCP3-binding domain. Various truncated SYCP2 polypeptides were cloned in the pACT2 vector and tested for interaction with full-length mouse SYCP3 in the pAS2-1 vector by yeast two-hybrid β-galactosidase filter assay. ++, dark blue within 3 h of incubation; +/−, light blue within 5 h; −, not blue after overnight incubation. Numbers indicate the position of terminal residues in each clone. The coiled coil region is shown as a black box. (C) In vitro GST pulldown assay. Full-length SYCP3 protein (254 aa) was expressed as a GST fusion protein and affinity purified. The SYCP2 polypeptides (residues 1,191–1,500) with or without the deletion (residues 1,346–1,476) were translated in vitro and tested for binding with GST-SYCP3. Arrows indicate the in vitro–translated SYCP2 polypeptides. Molecular mass standards are shown in kilodaltons.