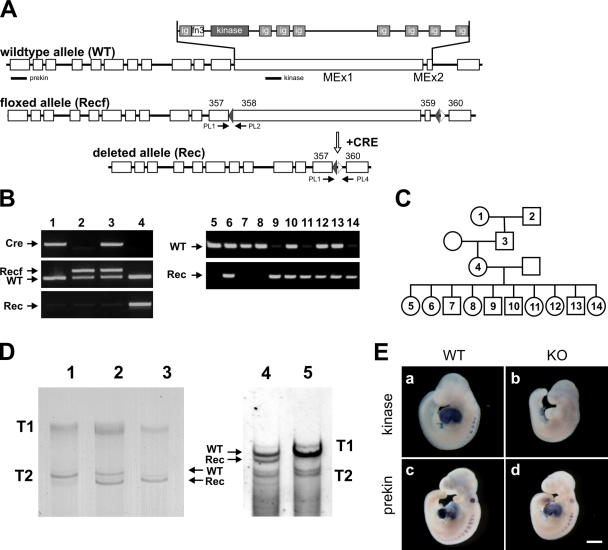
Figure 1.
Conversion of the inducible into a constitutive titin kinase region knockout. (A) Outline of the exon/intron structure of titin's M-line region and location of the genotyping primers (PL1, 2, and 4) and in situ probes (prekin and kinase). Cre-mediated recombination leads to the deletion of exons 358 and 359 (MEx1 and 2). (B) PCR-based genotyping of the protamine-Cre transgenic mouse (lane 1) and Recf mouse with loxP sites flanking MEx1 and 2 (lane 2) that were mated to obtain double heterozygotes (lane 3). After germline recombination, offspring contain the deleted Rec allele (lane 4). PCR analysis of embryos at E9.5 confirms early embryonic survival of homozygous knockouts (lanes 9, 11, and 14). (C) Pedigree for animals analyzed in B. (D) SDS-agarose gels of wild-type (lane 1), heterozygous (lane 2), and knockout hearts (lane 3) of E9.5 animals and adult heterozygous (lane 4) and wild-type animals (lane 5). Titin proteins of the predicted sizes are expressed in knockout animals. Because embryonic titin is expressed as a larger isoform, differences in migration are more prevalent in T2 titin. Truncated titin (Rec) is more stable in the embryo compared with adult heterozygotes (ratio of wild-type/knockout protein in lanes 2 and 4). Homozygous adults could not be obtained. (E) In situ hybridization of whole-mount E9.5 embryos using an antisense probe directed against the kinase region (a and b) and a probe that recognizes a region upstream of the kinase domain (prekin in c and d). Both heart and somites are stained in all controls, whereas the kinase probe in knockout animals does not produce a signal (b). Note the smaller body size of titin M-line knockout animals (KO). WT, wild type. Bar, 500 μm.