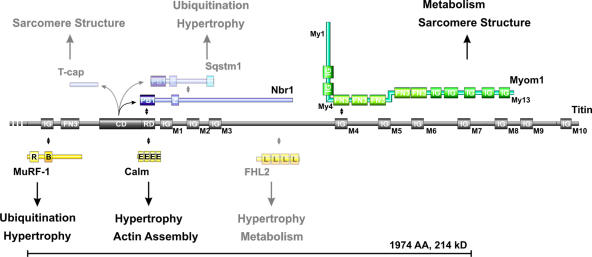
Figure 7.
Signaling and structural functions of titin's M-line region. The titin M-line region (gray) mainly consists of Ig domains labeled M1–M10, a fibronectin (FN3), and a kinase domain (CD + RD). The 214-kD region deleted in our titin M line–deficient animals (bracket) contains binding sites for MuRF-1, Nbr1, calmodulin, FHL2 (signaling proteins in yellow), and myomesin (green). Myomesin integrates into the M band through interaction with myosin (My1), titin (My4), and dimerization (My13). In vitro substrates for the truncated soluble kinase are T-cap, p62, and Nbr1 (blue). The titin M-line region has been proposed to cover structural (sarcomere assembly) as well as signaling functions (hypertrophy/atrophy) through its multiple binding partners. Proteins expressed at low levels or below the detection limit of our Western blot in the embryonic heart are semitransparent. Protein–protein interactions are indicated by double-pointed arrows. The protein domains depicted are PB1 (Phox and Bem1p domain), Z (zinc-binding domain), U (ubiquitin-associated domain), IG (Ig-like domain), FN3 (fibronectin type 3 domain), CD (titin kinase catalytic domain), RD (titin kinase regulatory domain), R (RING finger), B (B box–type zinc finger), E (EF hand calcium-binding motif), and L (LIM domain).