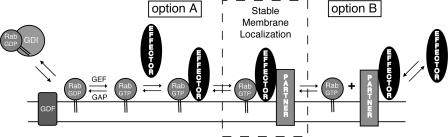
Figure 8.
Model for how effectors contribute to membrane localization of Rab proteins. GDP-bound Rabs are recruited to membranes from cytosolic complexes with GDI through the catalytic action of a GDF. A GEF catalyzes nucleotide exchange. Interaction of the Rab-GTP with an effector that has been recruited to the same membrane by a Rab (option A) or by an effector-binding partner (option B) will stabilize both the Rab and the effector on the membrane. If not bound to the effector, a GTPase-activating protein (GAP) can activate the Rab to hydrolyze its GTP to GDP, thus, allowing for potential membrane extraction of the Rab by GDI. Thus, the Rab GTPase is dependent on its effectors, and vice versa, for stable interaction with the membrane. The effector-binding partner need not be a protein and can be particular phospholipids, such as phosphoinositides, that are specific to a given compartment (Zerial and McBride, 2001).