Figure 4.
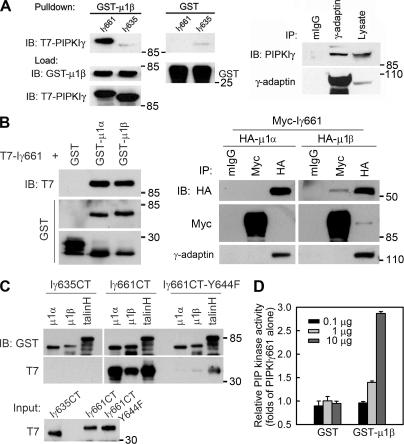
PIPKIγ661 associates with AP1B. (A, left) A GST pull-down assay was performed using 1 μg of each purified protein, and one fourth of the precipitates were analyzed by immunoblotting as indicated. A stock concentration of T7-tagged PIPKIγ661 and PIPKIγ635 was created for the GST pull-down assay. (right) A coimmunoprecipitation was performed and analyzed as indicated. MDCK cells from confluent 100-mm dishes were used to make 1-ml lysates for immunoprecipitation. All of each immunoprecipitate and 40 μl of each lysate were analyzed. (B, left) GST pull-down assays were performed using 1 μg of His-T7–tagged PIPKIγ or GST-fused proteins. (right) HEK293 cells were transfected with the indicated constructs using calcium phosphate. 48 h after transfection, cells were lysed and specific components were immunoprecipitated. The precipitates were analyzed by Western blot as indicated. The γ-adaptin subunit was coimmunoprecipitated with each of the HA-μ subunits, indicating that the overexpressed μ subunits were incorporated into AP1 complexes. However, when PIPKIγ was overexpressed, γ-adaptin association was weak; whereas in the reverse immunoprecipitation, association of PIPKIγ was readily detected. This could result from the competition of other PIPKIγ binding partners or from the sensitivity of the anti–γ-adaptin antibody. (C) GST pull-down assays were performed using 1 μg of His-T7–tagged indicated PIPKIγ C terminus or GST-fused proteins. The precipitates were analyzed by Western blot as indicated. (D) The indicated amount of purified GST or GST-μ1B was incubated with PIPKIγ at room temperature for 10 min before the kinase assay. Kinase activity was quantified from three independent experiments. Error bars are ± SEM.