Abstract
Cleavage of Notch by furin is required to generate a mature, cell surface heterodimeric receptor that can be proteolytically activated to release its intracellular domain, which functions in signal transduction. Current models propose that ligand binding to heterodimeric Notch (hNotch) induces a disintegrin and metalloprotease (ADAM) proteolytic release of the Notch extracellular domain (NECD), which is subsequently shed and/or endocytosed by DSL ligand cells. We provide evidence for NECD release and internalization by DSL ligand cells, which, surprisingly, did not require ADAM activity. However, losses in either hNotch formation or ligand endocytosis significantly decreased NECD transfer to DSL ligand cells, as well as signaling in Notch cells. Because endocytosis-defective ligands bind hNotch, but do not dissociate it, additional forces beyond those produced through ligand binding must function to disrupt the intramolecular interactions that keep hNotch intact and inactive. Based on our findings, we propose that mechanical forces generated during DSL ligand endocytosis function to physically dissociate hNotch, and that dissociation is a necessary step in Notch activation.
Introduction
Notch signaling regulates a diverse array of cell fates and cellular processes during embryonic development and contributes to adult homeostasis. Notch is a cell surface receptor that not only functions in ligand binding but is also the downstream signal transducer through a process of regulated intramembrane proteolysis (RIP; Brown et al., 2000). This mode of signaling depends on prior furin-mediated proteolysis to form an intramolecular, heterodimeric Notch (hNotch) receptor (Blaumueller et al., 1997; Logeat et al., 1998; Bush et al., 2001). The extracellular and membrane-bound intracellular furin-cleavage fragments of hNotch are held together through noncovalent interactions that prevent receptor activation in the absence of ligand (Rand et al., 2000; Sanchez-Irizarry et al., 2004). Binding of DSL (Delta/Serrate/Lag-2) ligands to hNotch activates signaling by inducing additional proteolysis, first within the Notch extracellular domain (NECD) via a disintegrin and metalloprotease (ADAM), which facilitates γ-secretase proteolysis within the membrane-spanning region to release the Notch intracellular domain (NICD; Brou et al., 2000; Mumm et al., 2000). Trans location of NICD to the nucleus allows it to interact with the DNA-binding protein CSL (CBF1, SuH, LAG-1) and recruit coactivators to activate transcription of Notch target genes (Wilkin and Baron, 2005).
Although activating proteases have been identified, the mechanism by which ligand binding leads to Notch proteolysis is still not well understood. DSL ligands, like Notch, are type 1 transmembrane proteins, and, accordingly, activation of Notch signaling requires direct cell–cell contact. Interestingly, endocytosis in the ligand cell is required to induce a signal in the Notch cell, suggesting additional roles beyond ligand presentation (Le Borgne et al., 2005; Wilkin and Baron, 2005; Chitnis, 2006). Studies in Drosophila melanogaster first suggested that ligand endocytosis of bound Notch promotes ADAM cleavage, leading to receptor dissociation and signaling (Parks et al., 2000). The exclusive uptake of the Notch ectodomain by Delta cells imaged in flies (Parks et al., 2000; Morel et al., 2003) is consistent with the idea that NECD sequences prevent receptor activation and must be removed before Notch can be proteolytically activated. Indeed, truncation of NECD sequences yields forms of Notch that are constitutively cleaved in the absence of ligand (Lieber et al., 1993; Rebay et al., 1993; Struhl et al., 1993; Schroeter et al., 1998). Furthermore, dissociation of mammalian hNotch via calcium chelators (Rand et al., 2000) or mutations within the heterodimerization domain mimics signaling induced by DSL ligands (Sanchez-Irizarry et al., 2004). That activating heterodimerization mutations are responsible for aberrant Notch signaling in T-cell acute lymphoblastic leukemia (Weng et al., 2004) provides additional support for Notch dissociation in receptor activation.
The NECD transendocytosis model for ligand activation of Notch is appealing; however, the ubiquitous expression of Notch makes it difficult to be certain that NECD imaged in Delta cells was actually donated by the neighboring Notch cell. Interpretation of such immunolocalization studies is further complicated by the exchange of full-length Delta and Notch between interacting cells (Klueg et al., 1998; Klueg and Muskavitch, 1999; Le Borgne and Schweisguth, 2003). Thus, the staining patterns could also represent internalization of cell surface Notch with Delta within the same cell, rather than transfer between cells.
To determine if activation of mammalian Notch signaling involves NECD transendocytosis, and to dissect the relative roles of endocytosis versus ADAM cleavage in receptor dissociation, we established a coculture system in which epitope-tagged forms of Notch permit direct visual confirmation for transfer of either NECD alone and/or intact Notch to DSL ligand cells. Our data reveal that after interaction with Notch cells, DSL ligand cells preferentially take up NECD, whereas the bulk of the NICD remains in the receiving cell, where it undergoes proteolytic activation. In addition, both receptor dissociation and internalization of NECD by DSL ligand cells require hNotch formation by furin processing, yet occur independent of ADAM proteolysis. Based on our findings, we conclude that hNotch must first dissociate before activating Notch proteolysis can occur, and we propose that DSL ligand endocytosis participates in the physical dissociation of Notch, rather than promoting enzymatic dissociation as previously suggested (Parks et al., 2000).
Results
DSL ligands induce clustering and transendocytosis of Notch1
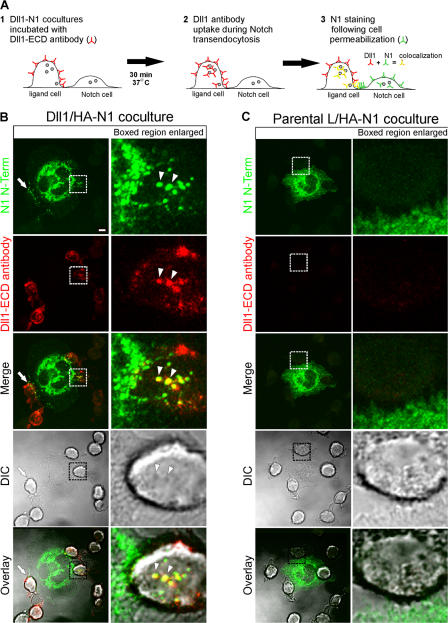
To unambiguously determine if Notch signaling in mammalian cells also involves transendocytosis of NECD by interacting ligand cells, and to circumvent the problems associated with ubiquitous expression of Notch, we engineered epitope-tagged forms of Notch1 (N1) and stably expressed them in C2C12 cells for use in coculture studies. We have previously reported that L cells stably expressing DSL ligands (Delta-like1 [Dll1] or Jagged1 [J1]) activate Notch signaling when cocultured with N1-expressing cells (Lindsell et al., 1995; Hicks et al., 2000; Yang et al., 2005). Based on these studies, Dll1 cells were cocultured with cells expressing an N-terminal HA-tagged N1 (HA-N1) in the presence of rabbit polyclonal antibodies to the Dll1 extracellular domain (148G), as outlined in Fig. 1 A. After incubation, the cells were fixed, permeabilized, and stained with both a fluorescently conjugated mouse monoclonal HA antibody, to identify HA-N1, and a fluorescently conjugated anti–rabbit antibody, to detect surface and internalized Dll1. Confocal imaging revealed HA puncta polarized toward Dll1 cells at sites of cell–cell contact, producing a nonuniform punctate expression pattern for N1 within cell membranes (Fig. 1 B, arrows). Importantly, this ligand-induced receptor clustering and nonuniform punctate expression pattern was not detected for HA-N1 cells in direct contact with parental L cells (Fig. 1 C). Moreover, the acquired images suggest that HA and Dll1 signals colocalize within vesicular structures of Dll1 cells (Fig. 1 B, arrowheads).
Figure 1.
DSL ligands induce clustering and transendocytosis of Notch1. (A) Coculture and staining protocol for Dll1 endocytosis and N1 transendocytosis (see Materials and methods for details). Small filled circles represent intracellular vesicles. (B and C) HA-N1 C2C12 cells were cocultured with Dll1 (B) or parental L (C) cells in the presence of rabbit anti-Dll1 extracellular domain (ECD) antibodies to track Dll1 internalization. Cells were then fixed, permeabilized, stained with an HA antibody (16B12) conjugated to Alexa Fluor 488 to detect N1 N terminus (green) and anti–rabbit Alexa Fluor 633 to detect Dll1 antibodies (red), and imaged by confocal and differential interference contrast (DIC) microscopy. Arrows indicate N1 puncta polarized at interfaces of Dll1–N1 cells; arrowheads indicate colocalization of N1 and Dll1 within the Dll1 cell (yellow). Boxes indicate enlarged regions. Overlays are composites of fluorescent and DIC images. Images were uniformly adjusted using the levels function in Photoshop (Adobe). Bar, 5 μm.
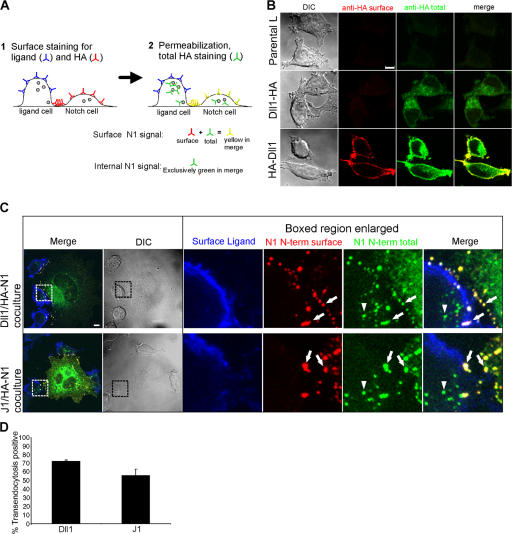
Although confocal sectioning suggested that the HA-Dll1–positive vesicles were intracellular (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200609014/DC1), we designed a staining protocol (Fig. 2 A) to distinguish HA staining at the surface from that located inside Dll1 cells. In support of this staining protocol, cells expressing a C-terminal HA-tagged Dll1 showed a signal only after permeabilization, whereas an extracellular HA-tagged Dll1 was detected by staining with HA antibodies both before and after permeabilization (Fig. 2 B). Given this validation, cocultures stained in this manner revealed signals for Dll1 and HA-N1 at the cell surface (Fig. 2 C, arrows), whereas permeabilization and staining with the second HA antibody identified a vesicular signal within Dll1 cells (arrowheads), which is consistent with transfer of HA-N1 to Dll1 cells. Similar staining patterns were also observed for HA-N1 cells cocultured with J1 cells (Fig. 2 C), suggesting that Notch transendocytosis is a general phenomenon of DSL ligands (Fig. 2 D), as previously reported for the two D. melanogaster ligands, Delta and Serrate (Klueg and Muskavitch, 1999).
Figure 2.
Transendocytosed Notch1 structures are internal and disconnected from the plasma membrane. (A) Staining protocol to distinguish surface and internal N1 (see Materials and methods for details). (B) Validation of protocol in A. L cells expressing Dll1 with HA tags on either the intracellular (Dll1-HA) or extracellular (HA-Dll1) domain were fixed and stained for surface HA with mouse HA antibody (262K) and anti–mouse Alexa Fluor 568 (red). After permeabilization, cells were stained for total HA (surface and intracellular) with an HA antibody (16B12) conjugated to Alexa Fluor 488 (green) and imaged by confocal and DIC microscopy. (C) Cocultures of HA-N1 cells with Dll1 or J1 cells were fixed and stained with rabbit anti-ECD antibodies to Dll1 or J1 and anti–rabbit Alexa Fluor 633 antibodies (blue) to label the surface of the ligand cell, followed by staining for surface N1 N terminus with a mouse HA antibody (262K) and anti–mouse Alexa Fluor 568 (red). After permeabilization, cells were stained for total N1 N terminus (surface and intracellular) with an HA antibody (16B12) conjugated to Alexa Fluor 488 (green), and imaged by confocal and DIC microscopy. Arrows indicate N1 N terminus on both the Notch cell surface (yellow) and the ligand cell surface (white). Arrowheads indicate internal N1 N terminus detected within ligand cells (green). Boxes denote enlarged regions. (D) Transendocytosis was quantified by examining Dll1 or J1 cells for N1 N terminus detected exclusively after permeabilization (see Materials and methods). Error bars represent the SEM. Images from each experiment were uniformly adjusted using the levels function in Photoshop. Bars, 5 μm.
Transfer of Notch to DSL ligand cells correlates with activating proteolysis
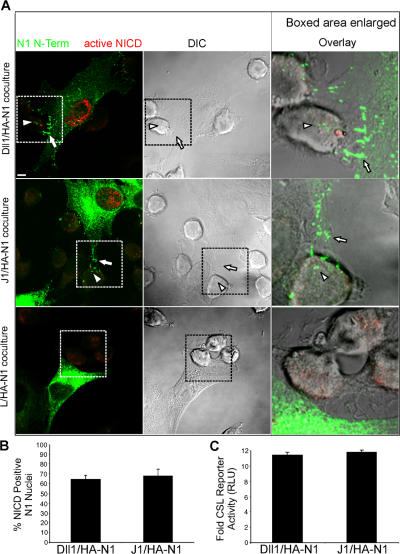
Dissociation of Notch by DSL ligand cells in D. melanogaster has been linked to signaling, but its requirement in activating proteolysis has not been directly demonstrated. To address this question, cocultures were stained with both an antibody specific for the γ-secretase–cleaved active NICD (Val1744), as well as for HA antibodies to mark the N1 N-terminal tag. To enhance detection of active NICD, proteosome inhibitors were used to prevent its rapid turnover (Wu et al., 2001). As discussed in the previous section, ligand cells in direct contact with HA-N1 cells typically induced a polarized punctate HA signal at sites of cell–cell contact (Fig. 3 A, arrows), which is indicative of ligand-induced receptor clustering. Such interacting cells were then scored for HA puncta associated with ligand cells (Fig. 3 A, arrowheads), which is suggestive of HA-N1 transfer, as well as a signal for active NICD in the nucleus of HA-N1 cells, which is indicative of Notch signaling. This analysis revealed that 60–70% of these interacting cells displayed both a punctate HA signal associated with ligand cells and a signal for active NICD in the nucleus of HA-N1 cells (Fig. 3 B). Receptor clustering and transendocytosis by DSL ligand cells, as well as a signal for active NICD in the nuclei of HA-N1 cells, all correlated with activation of a Notch reporter in coculture assays (Fig. 3 C). In contrast, HA-N1 cells in direct contact with parental L cells did not display polarized HA-positive puncta or a signal for active NICD, and the interacting L cells were negative for HA staining (Fig. 3 A). In addition, cells expressing an N1 protein tagged at its C terminus with EGFP displayed signals for both EGFP and active NICD in the nucleus that were specific for interactions with Dll1 cells (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200609014/DC1). Together, our findings suggest that DSL ligand cells interact with Notch cells to induce receptor clustering and transendocytosis, and that these events promote Notch proteolysis and downstream signaling. Importantly, this coculture system has provided direct support for N1 transendocytosis by DSL ligand cells, and has allowed us to investigate the requirements for transfer of Notch to ligand cells, as well as the relevance of such transfer to signaling.
Figure 3.
DSL ligands mediate clustering and transendocytosis of Notch1 to activate Notch1 proteolysis and downstream signaling. (A) HA-N1 cells cocultured with Dll1, J1, or parental L cells were fixed, permeabilized, and stained with an HA antibody (16B12) conjugated to Alexa Fluor 488 to detect the N1 N terminus (green), followed by rabbit antibodies to activated NICD (Val1744) and anti–rabbit Alexa Fluor 568 (red) and imaged by confocal and DIC microscopy. Arrows indicate N1 N terminus polarized to sites of contact between ligand and N1 cells. Arrowheads indicate N1 N terminus within ligand cells interacting with N1 cells and displaying a signal for activated NICD in the nucleus. Boxes denote enlarged regions; overlays are composites of fluorescent and DIC images. A low level of red-channel nonnuclear background fluorescence that is insensitive to DAPT (Fig. 5 E) is detected with all L-cell lines. (B) Cells displaying transfer of N-terminal puncta to Dll1 or J1 cells were scored for NICD-positive nuclei. (C) HA-N1 cells transfected with a CSL-luciferase reporter were cocultured with ligand cells and assayed for luciferase activity. Values represent fold-induction over cocultures with parental L cells. RLU, relative luciferase units. Error bars represent the SEM (B) and the SD (C). Images were uniformly adjusted using the levels function in Photoshop. Bar, 5 μm.
NECD is preferentially transferred to DSL ligand cells
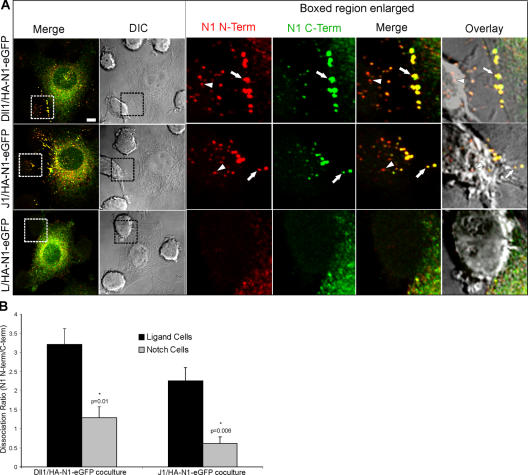
The intracellular HA-positive vesicular signal detected for ligand cells in direct contact with HA-N1 cells (Fig. 1 and 2) could be caused by the transfer of the NECD alone and/or the entire N1 protein. In D. melanogaster, transendocytosis of full-length Delta and Notch, as well as a multipass membrane protein, BOSS (Cagan et al., 1992), has been reported. In mammalian systems, transendocytosis of full-length ephrins and EPH receptors is important for biological effects mediated by this signaling system (Marston et al., 2003; Zimmer et al., 2003). However, because we find that transfer of N1 to ligand cells correlates with a signal for active NICD in interacting N1 cells, transfer of the N-terminal subunit exclusive of its membrane-bound C-terminal subunit must occur. To directly address the relative levels of NECD versus full-length N1 transfer, we examined cells expressing an N-terminal HA and C-terminal EGFP-tagged N1 protein (HA-N1-EGFP). Signals for both tags colocalized to large puncta at the N1 cell periphery and within membrane extensions polarized toward ligand cells (Fig. 4 A, arrows and overlay). In contrast, vesicular structures associated with ligand cells were primarily positive for the N-terminal tag (Fig. 4 A, arrowheads and overlay), which is indicative of NECD in the absence of the C-terminal subunit. To quantitate the transfer of NECD versus full-length N1 for both ligand and N1 cells, we determined the dissociation ratio, which is a measure of the HA–N-terminal tag relative to that detected for the EGFP–C-terminal tag (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200609014/DC1; see Materials and methods). We reasoned that if NECD was being removed from the N1 cell and transferred to the ligand cell then the dissociation ratio calculated for ligand cells should be greater than that determined for N1 cells, where similar levels of both tags would be detected if the receptor remained intact. The dissociation ratio was higher for ligand cells compared with N1 cells (Fig. 4 B), suggesting that the majority of the transendocytosed signal represents transfer of NECD independent of NICD.
Figure 4.
DSL ligand–induced transendocytosis promotes separation of the Notch1 N- and C-terminal subunits. (A) HA-N1-EGFP C2C12 cells were cocultured with Dll1, J1, or parental L cells. Cocultures were fixed, permeabilized, and stained with a mouse HA antibody (262K) and anti–mouse Alexa Fluor 568 to detect the N1 N terminus (red) and a rabbit anti-GFP antibody conjugated to Alexa Fluor 488 to detect the N1 C terminus (green), and then imaged by confocal and DIC microscopy. Arrows indicate double-positive HA-N1-EGFP clusters at interfaces between N1 and ligand cells. Arrowheads indicate puncta positive only for the N1 N terminus within ligand cells. Boxes denote enlarged regions; overlays are composites of fluorescent and DIC images. (B) The signals for Notch N and C termini were divided to produce the dissociation ratio (see Materials and methods) for ligand and Notch cells. Error bars represent the SEM. *, P < 0.05; t test relative to ligand cells. Images were uniformly adjusted using the levels function in Photoshop. Bar, 5 μm.
Notch heterodimer dissociation does not require ADAM proteolysis
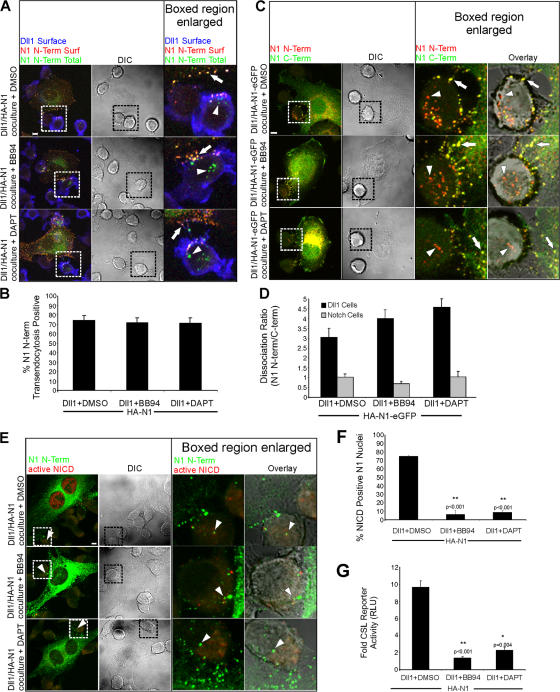
Transendocytosis of NECD alone by ligand cells may depend on ADAM cleavage of Notch. Alternatively, ADAM proteolysis may require receptor dissociation and NECD removal. To distinguish between these possibilities, we examined the effect of inhibiting metalloproteases with BB94 on HA-N1 transendocytosis and receptor dissociation. Specific staining to identify intracellular HA signals identified signals within Dll1 cells for both BB94 and DMSO control cocultures (Fig. 5, A and B; arrowheads). Examination of cells expressing a double-tagged HA-N1-EGFP identified Dll1 cells with HA signals in the absence of EGFP signals (Fig. 5 C; arrowheads), which was not the case for Notch cells. Consistent with this, the dissociation ratio determined for Dll1 cells compared with Notch cells was considerably higher (Fig. 5 D). Importantly, BB94 treatment did not alter the dissociation ratios, suggesting that metalloprotease inhibition does not perturb the separation and removal of the N-terminal tag from its C-terminal tag on HA-N1-EGFP. Despite detection of NECD transendocytosis, NICD generation (Fig. 5, E and F) and Notch reporter activity (Fig. 5 G) were reduced by the addition of BB94, underscoring the importance of ADAM proteolysis in Notch activation. Although ADAM proteolysis is required for NICD generation, our findings provide the first evidence that ADAM proteolysis does not function in receptor dissociation. Not surprisingly, the γ-secretase inhibitor DAPT prevented a signal for activated NICD (Fig. 5, E and F) and reporter activity (Fig. 5 G); however, NECD transendocytosis (Fig. 5, A and B) and separation of the N and C termini (Fig. 5, C and D) were unaffected. Together, our findings suggest that NECD release and transendocytosis mediated by Dll1 cells precede and facilitate Notch proteolysis, rather than being the results of ADAM cleavage, as previously suggested.
Figure 5.
Notch1 transendocytosis and N-terminal dissociation does not require cleavage by ADAM or γ-secretase. (A) Dll1 cells were cocultured with HA-N1 cells in the presence of vehicle control (DMSO), ADAM inhibitor (BB94), or γ-secretase inhibitor (DAPT). Cocultures were treated as in Fig. 2 (A and C) to identify surface Dll1 (blue), surface N1 N terminus (red), and total N1 N terminus (green). Arrows indicate N1 N terminus on the surface of the Notch cells (yellow). Arrowheads indicate N1 N terminus detected within Dll1 cells (green). (B) Transendocytosis was quantified as in Fig. 2 D. (C) Dll1 cells were cocultured with HA-N1-EGFP cells in the presence of DMSO, BB94, or DAPT, as in Fig. 4 A, to identify N1 N terminus (red) and N1 C terminus (green). Arrows indicate double-positive N1 clusters (yellow); arrowheads denote vesicular structures positive for only N1 N terminus (red). (D) Dissociation ratios were quantified for both Dll1 and Notch cells, as in Fig. 4 B. (E) Dll1 cells were cocultured with HA-N1 cells in the presence of DMSO, BB94, or DAPT, as in Fig. 3 A, to identify N1 N terminus (green) and activated NICD (red). Arrowheads indicate N1 N terminus within Dll1 cells interacting with N1 cells. (F) NICD-positive nuclei were scored as in Fig. 3 B. (G) CSL reporter assay in the presence of DMSO, BB94, or DAPT as in Fig. 3 C. Cocultures in (A, C, and E) were imaged by confocal and DIC microscopy. Boxes indicate enlarged regions. Overlays are composites of fluorescent and DIC images. Error bars represent the SEM (B, D, and F) and the SD (G). *, P < 0.05; **, P < 0.001; t test relative to DMSO controls. Images from each experiment were uniformly adjusted using the levels function in Photoshop. Bars, 5 μm.
NECD transendocytosis is dependent on Notch1 heterodimer formation
Because ADAM proteolysis was not required for NECD transendocytosis, we reasoned that the physical dissociation of NECD to Dll1 cells might rely on hNotch1 formation that requires furin processing (Logeat et al., 1998; Bush et al., 2001). To investigate this, we engineered N- and C-terminal–tagged versions of a previously described N1 mutant defective in furin processing and hNotch1 formation (Bush et al., 2001). Importantly, this mutational approach should specifically block NECD transendocytosis without affecting ligand endocytosis, and determine if specific transendocytosis of NECD is required for signaling.
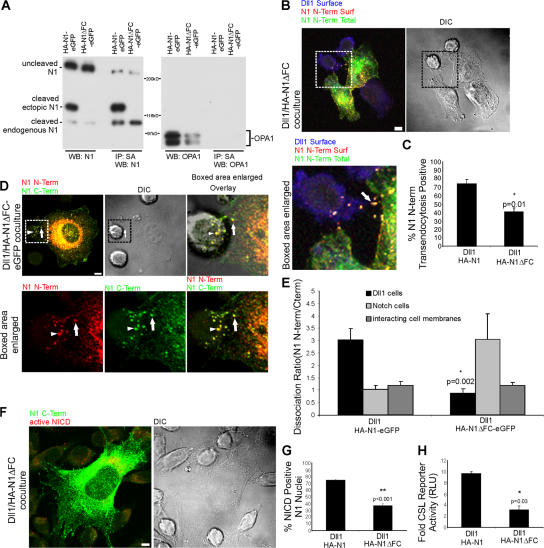
Western blot analysis of HA-N1-EGFP–expressing cells identified cleaved forms of both endogenous and ectopic N1 proteins, the latter having decreased mobility because of its C-terminal EGFP tag (Fig. 6 A). Although HA-N1ΔFC-EGFP cells express cleaved endogenous N1, a cleaved ectopic N1 was not detected. Importantly, surface labeling with biotin identified both uncleaved and cleaved forms of HA-N1-EGFP, whereas only an uncleaved form was detected on the surface of HA-N1ΔFC-EGFP cells (Fig. 6 A). This protein analysis confirms that the mutant HA-N1ΔFC-EGFP protein is not proteolytically processed into a heterodimeric form, but instead exists at the cell surface as an uncleaved receptor.
Figure 6.
Notch1 transendocytosis, N-terminal dissociation, and signaling require furin processing. (A) C2C12 cells stably expressing HA-N1-EGFP or the furin cleavage mutant HA-N1ΔFC-EGFP were biotinylated, and lysates were quantitated and equilibrated. Whole cell lysates, as well as streptavidin precipitates, were analyzed by Western blotting with antibodies to N1 intracellular domain (93–4), or OPA1. Uncleaved N1, as well as the C-terminal, 120-kD, furin-processed subunit of both ectopic and endogenous N1, are detected. (B) Dll1 cells were cocultured with HA-N1ΔFC cells, as in Fig. 2 C, to identify surface Dll1 (blue), surface N1 N terminus (red), and total N1 N terminus (green). Arrow indicates N1 N terminus at the interface of Notch and Dll1 cells. (C) Transendocytosis was quantified as in Fig. 2 D. (D) Dll1 cells were cocultured with HA-N1ΔFC-EGFP as in Fig. 4 A to identify N1 N terminus (red) and N1 C terminus (green). Arrows indicate double-positive N1 clusters at the interface of Notch and Dll1 cells; arrowheads denote double-positive structures associated with Dll1 cells. (E) Dissociation ratio was quantified for Dll1 cells, Notch cells, and the interacting cell membranes as in Fig. 4 B. (F) Dll1 cells were cocultured with HA-N1ΔFC cells and treated as in Fig. 3 A to identify N1 N terminus (green) and activated NICD (red). (G) NICD-positive nuclei were scored as in Fig. 3 B. (H) CSL reporter assay with HA-N1 or HA-N1ΔFC cells, as in Fig. 3 C. Cocultures in B, D, and F were imaged by confocal and DIC microscopy. Boxes indicate enlarged regions. Overlays are composites of fluorescent and DIC images. Error bars represent the SEM (C, E, and G) and the SD (H). *, P < 0.05, **, P < 0.001; t test relative to wild-type N1. Images from each experiment were uniformly adjusted using the levels function in Photoshop. Bars, 5 μm.
Imaging of cells stably expressing HA-N1ΔFC cocultured with Dll1 cells revealed clusters of HA-positive puncta at borders of interacting cells (Fig. 6 B, arrow), indicative of ligand–receptor interactions. Despite this ligand-induced receptor clustering, detection of the HA–N-terminal signal within interacting Dll1 cells was significantly reduced, but not completely eliminated (Fig. 6 C). However, given that this particular analysis detects only the N-terminal tag, it is possible that the signal represents internalization of intact HA-N1ΔFC. To investigate this, we examined cells expressing a double-tagged HA-N1ΔFC-EGFP, and found puncta positive for both tags within N1 cell membranes (Fig. 6 D, arrows and overlay), as well as associated with Dll1 cells (arrowheads), which is suggestive of the transfer of intact receptor. Consistent with this, the HA-N1ΔFC-EGFP dissociation ratio calculated for Dll1 cells was close to 1.0, reflecting an equivalent transfer of both tags (Fig. 6 E). EGFP fluorescence calculated for the entire mutant-expressing cell was decreased compared with the N-terminal tag (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200609014/DC1), resulting in a dissociation ratio >1.0; however, similar dissociation ratios were calculated for wild-type– and mutant-tagged proteins within interacting cell membranes (Fig. 6 E), and both tags were congruent at the cell surface, where interactions with ligand cells would occur (compare Fig. 5 C and Fig. 6 D, arrows). It is possible that conformational changes induced by the deletion alter detection of the C-terminal tag on proteins within the cell.
The losses in dissociation calculated for HA-N1ΔFC-EGFP cells correlate with significant losses in active NICD and reporter activity (Fig. 6, F–H). The signaling detected with HA-N1ΔFC-EGFP cells is likely caused by activation of endogenous N1 (Fig. 6 A). Together, our analysis of the furin cleavage mutant indicates that losses in hNotch formation are associated with significant losses in receptor dissociation and signaling, indicating that heterodimer formation is required for NECD transendocytosis by ligand cells, and that this event is a prerequisite for Notch activation.
NECD transendocytosis and Notch activation require Dll1 endocytosis
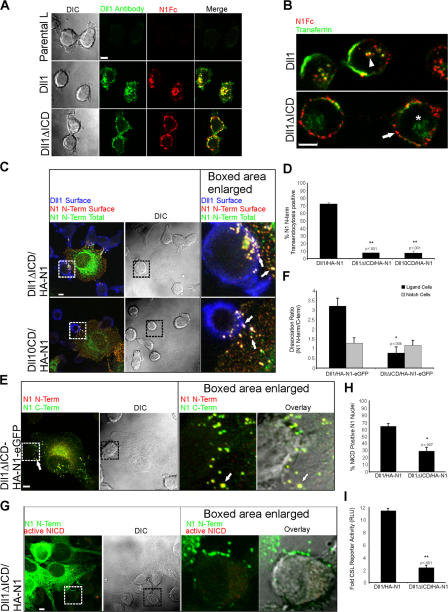
Our data indicate that NECD transendocytosis occurs independent of ADAM proteolysis, but requires a furin-processed heterodimer. Given the obligatory role of dynamin-dependent endocytosis in Notch signaling (Seugnet et al., 1997), we next asked if Dll1 endocytosis might contribute to heterodimer dissociation. For these studies, we generated Dll1 mutants lacking most (Dll1ΔICD), or all (Dll10CD), intracellular sequences because these sequences are required for Delta endocytosis (Chitnis, 2006). Removal of Dll1 intracellular sequences prevented uptake of Dll1 extracellular antibodies, as well as a soluble form of N1 (N1Fc; Fig. 7 A). However, internalization of transferrin by Dll1ΔICD cells was unaffected (Fig. 7 B), indicating that the block in endocytosis is Dll1 specific.
Figure 7.
Notch1 transendocytosis, hNotch1 subunit separation, and signaling require Dll1 endocytosis. (A) Dll1, Dll1ΔICD, or parental L cells were incubated with rabbit Dll1 extracellular domain (ECD) antibodies and N1Fc to track Dll1. After incubation, the cells were fixed, permeabilized, and stained with anti–rabbit Alexa Fluor 488 to detect Dll1 antibodies (green) and anti–human Fc conjugated to Cy5 (red) to detect N1Fc. (B) Dll1 or Dll1ΔICD cells were incubated with N1Fc preclustered with anti–human Fc conjugated to Texas red (red), and transferrin conjugated to FITC (green). Arrowhead indicates internalized N1Fc colocalized with transferrin in Dll1 cells (yellow). Arrow indicates N1Fc on the surface of Dll1ΔICD cells (red). Asterisk indicates internalized transferrin. (C) Cocultures of HA-N1 cells and Dll1ΔICD or Dll10CD cells were treated as in Fig. 2 C to identify surface mutant Dll1 (blue), surface N1 N terminus (red), and total N1 N terminus (green). Arrows indicate N1 N terminus on the surface of the Notch cell (yellow), as well as the mutant ligand cell (white). (D) Transendocytosis was quantified as in Fig. 2 D. (E) Cocultures of HA-N1-EGFP and Dll1ΔICD cells were treated as in Fig. 4 A to identify N1 N terminus (red) and N1 C terminus (green). Arrow indicates double-positive HA-N1-EGFP cluster (yellow). (F) Dissociation ratio was quantified as in Fig. 4 B. (G) Dll1ΔICD cells were cocultured with HA-N1 cells and treated as in Fig. 3 A to identify N1 N terminus (green) and activated NICD (red). (H) NICD-positive nuclei were scored as in Fig. 3 B. (I) CSL reporter assay, as in Fig. 3 C. Cells in A–C, E, and G were imaged by confocal and DIC microscopy. Boxes indicate enlarged regions. Overlays are composites of fluorescent and DIC images. *, P < 0.05; **, P < 0.001; t test relative to Dll1 cells. Error bars represent the SEM (D, F, and H) and the SD (I). Images from each experiment were uniformly adjusted using the levels function in Photoshop. Bars, 5 μm.
Endocytosis-defective Dll1ΔICD and Dll10CD cells induced clustering of tagged forms of N1 at sites of cell–cell contact (Fig. 7 C, arrows); however, both NECD transendocytosis (Fig. 7, C and D) and the ligand cell dissociation ratio were significantly reduced compared with full-length Dll1 (Fig. 7, E and F), indicating that ligand-mediated endocytosis of Notch is required to separate the N- and C-terminal subunits. Importantly, losses in NECD transendocytosis associated with Dll1ΔICD and Dll10CD cells correlate with a decrease in NICD-positive nuclei (Fig. 7, G and H; not depicted) and reporter activity (Fig. 7 I; not depicted), highlighting a critical requirement for Dll1-specific endocytosis in Notch signaling, as well as a specific role for ligand activity beyond receptor binding and clustering.
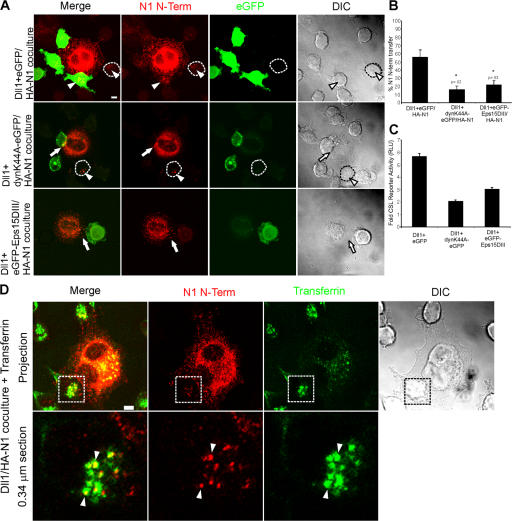
To directly address a requirement for ligand cell endocytosis in NECD transendocytosis, we transiently expressed mutant forms of dynamin (dynk44A-EGFP; Damke et al., 1994) or Eps15 (EGFP-Eps15DIII; Benmerah et al., 1998) in Dll1 cells because these constructs are known to inhibit endocytosis. Expression of either dynk44A-EGFP or EGFP-Eps15DIII suppressed transfer of HA-N1 to Dll1 cells (Fig. 8, A and B) and reduced ligand-induced Notch signaling (Fig. 8 C). Consistent with the losses in NECD transfer, when either dynamin or Eps15 activities were inhibited, NECD internalized by Dll1 cells overlapped with transferrin, a known cargo of clathrin-mediated endocytosis that requires both dynamin and Eps15 (Fig. 8 D). Given that perturbation of either dynamin or Eps15 activity did not decrease Dll1 cell surface expression or N1Fc binding (Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200609014/DC1; not depicted), the losses in reporter activity (Fig. 8 C) indicate that NECD transendocytosis by Dll1 cells is required to generate a signal in interacting Notch1 cells. If hNotch dissociation is driven by ligand endocytosis, and if both events are required for Notch proteolysis then this could explain, in part, the requirement for DSL ligand endocytosis in Notch signaling.
Figure 8.
Notch1 transendocytosis and signaling require general endocytic machinery. (A) Dll1 cells were transiently transfected with EGFP, dynaminK44A-EGFP, or EGFP-Eps15DIII, and then cocultured with HA-N1 cells. Cocultures were fixed, permeabilized, and stained with a mouse HA antibody (262K) and anti–mouse conjugated to Alexa Fluor 568 to detect the N1 N terminus (red), followed by rabbit anti-GFP conjugated to Alexa Fluor 488 to detect EGFP (green). Arrows indicate interacting N1 N terminus. Arrowheads indicate N1 N terminus associated with Dll1 cells. An untransfected Dll1 cell is outlined. Fluorescent images are confocal projections through the midsection of the Dll1 cell. (B) Transfer of N1 N terminus was quantified by examining Dll1 cells displaying EGFP fluorescence for internal N1 N terminus (see Materials and methods). (C) HA-N1 cells transfected with a CSL-luciferase reporter were cocultured with HEK 293-T cells cotransfected with Dll1 and EGFP, dynaminK44A-EGFP, or EGFP-Eps15DIII and assayed for luciferase activity. Values are fold-induction over vector + EGFP-transfected cells. (D) HA-N1 cells were cocultured with Dll1 cells in the presence of transferrin conjugated to FITC (green). Cocultures were fixed, permeabilized, and stained with a mouse HA antibody (262K) and anti–mouse conjugated to Alexa Fluor 568 to detect the N1 N terminus (red). Arrowheads indicate colocalization of transferrin and N1 N terminus in Dll1 cells (yellow). Boxes denote enlarged region. Low magnification fluorescent images are confocal projections, and enlargements are a 0.34 μm confocal section through the midsection of the Dll1 cell. Error bars represent the SEM (B) and the SD (C). *, P < 0.05; t test relative to Dll1+EGFP cells. Images from each experiment were uniformly adjusted using the levels function in Photoshop. Bars, 5 μm.
DSL ligand-mediated receptor dissociation precedes and permits activating Notch proteolysis
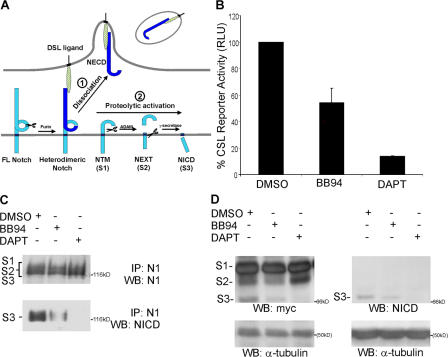
Our findings suggest a two-step model in which ligand endocytosis induces nonenzymatic dissociation of bound Notch to allow uptake of NECD by ligand cells (Fig. 9 A, step 1). The remaining, membrane-bound hNotch subunit (NTM/S1) would undergo constitutive cleavage by ADAM and γ-secretase to produce NEXT/S2 and NICD/S3, respectively (Fig. 9 A, step 2). In support of this model, we found that a recombinant NTM/S1 form was constitutively active in a reporter assay, and that BB94 reduced the level of signaling (Fig. 9 B), indicating that ADAM cleavage is required for maximal activity. Moreover, losses in active NICD/S3 (detected by the Val1744 antibody) produced by BB94 and DAPT provide additional evidence for constitutive cleavage of recombinant S1 by both ADAM and γ-secretase, respectively (Fig. 9 C). Nonetheless, to directly demonstrate that S1 undergoes ADAM cleavage to produce the transient S2 form, it was necessary to truncate and myc tag recombinant S1 to resolve the S1, S2, and S3 forms by SDS-PAGE. After expression in COS cells, both S2 and S3 cleavage products were detected (Fig. 9 D); however, ADAM inhibition decreased the appearance of S2 and S3, whereas DAPT blocked the appearance of S3 and lead to the accumulation of S2, indicating that S1 is an ADAM substrate (Brou et al., 2000; Mumm et al., 2000). Together these findings are consistent with our model that after hNotch1 dissociation, membrane-bound S1 is constitutively cleaved first by ADAM, and then by γ-secretase.
Figure 9.
A two-step model for Notch activation: DSL ligand endocytosis dissociates Notch heterodimers and permits activating proteolysis. (A) After ligand binding to Notch, DSL ligand-mediated endocytosis nonenzymatically dissociates the Notch heterodimeric subunits (1). Physical removal of NECD by transendocytosis exposes the remaining membrane-bound NTM subunit to ADAM and γ-secretase proteolysis for release of NICD (2). (B) COS7 cells were transfected with either a truncated NTM-like construct (p120mis) or vector control and a CSL-luciferase reporter construct, and then incubated in the presence of DMSO, BB94, or DAPT. Luciferase activity is shown as a percentage of the DMSO control activity of p120mis. The average p120mis activity was 23-fold over the vector control. (C) COS7 cells were transiently transfected with an NTM-like construct (p120mis) and cultured in the presence of DMSO, BB94, or DAPT, as well as a proteosome inhibitor (MG132). Cell lysates were immunoprecipitated with rabbit antibodies to N1 intracellular domain (PCR12), followed by Western blot analysis with antibodies to N1 intracellular domain (93–4; top) or rabbit antibodies to activated NICD (Val 1744; bottom). (D) A truncated NTM-like construct, p120misΔmyc, was transiently transfected into COS7 cells and cultured in the presence of DMSO, BB94, or DAPT, as well as a proteosome inhibitor (MG132). Cell lysates were analyzed by Western blotting with myc (9E10) or NICD (Val1744) antibodies, and detection of α-tubulin was used as a loading control.
Discussion
Transendocytosis of NECD by DSL ligand cells results in Notch proteolytic activation
The phenomenon of Notch transendocytosis by ligand cells has been described in D. melanogaster and linked to Notch signaling (Parks et al., 2000), yet the mechanism and relevance of Notch uptake by ligand cells to Notch activation remains controversial (Chitnis, 2006). In fact, Notch transendocytosis via interacting ligand cells has been suggested to simply reflect clearance of the ADAM-shed Notch ectodomain, rather than directly affecting Notch proteolysis (Wilkin and Baron, 2005).
To explore the idea that NECD transendocytosis represents a critical step in Notch activation, we developed a system that employs dual epitope-tagged proteins to directly image the transfer of NECD to interacting ligand cells. Our results both confirm and extend the studies in D. melanogaster, where distinct cellular staining patterns for the Notch extracellular and intracellular domains were interpreted to represent dissociation of the Notch receptor after ligand activation (Klueg et al., 1998; Klueg and Muskavitch, 1999; Parks et al., 2000). We found that mammalian N1 receptors were clustered by, transferred to, and taken up by ligand cells, and that these events correlate with detection of cleaved, active NICD in the nucleus of contacted Notch cells. We conclude that ligand activation of Notch involves separation of the heterodimer, and that this allows translocation of the dissociated NECD to the ligand cell and of the cleaved NICD to the nucleus of the Notch cell.
NECD transendocytosis occurs before ADAM proteolysis
The dependence of NECD transendocytosis on functional ligand endocytosis first suggested that ligand internalization of bound Notch imparts a molecular strain to induce conformational changes that allow ADAM cleavage within the NECD (Parks et al., 2000). However, other studies have suggested that ADAM cleavage is independent of ligand endocytosis, and that activating γ-secretase cleavage occurs only after the ADAM-cleaved NECD is removed by endocytosis (Shimizu et al., 2002). We initially favored the idea that removal of NECD from the intact Notch receptor would require ADAM proteolysis, but we found that metalloprotease inhibition did not impair heterodimer dissociation or NECD transendocytosis. Although we cannot rule out that proteases other than metalloproteases might cleave NECD, our findings indicate that Notch dissociation and transendocytosis occur independently of, and before, ADAM cleavage, rather than arise as a consequence of proteolysis. Additional support for this idea comes from our demonstration that the secreted extracellular matrix proteins microfibril-associated glycoprotein 1 and 2 activate Notch signaling through hN1 dissociation, independent of ADAM proteolysis (Miyamoto et al., 2006).
Heterodimeric dissociation is a prerequisite for proteolytic activation
Our data indicate that ADAM activity is not required for NECD transendocytosis, but we also find that ADAM activity is absolutely necessary for Notch signaling induced by DSL ligands. Furthermore, we found that forms of N1 defective in heterodimer formation were reduced in both NECD-specific transendocytosis and separation of the N- and C-terminal tags after contact with Dll1 cells. Moreover, losses in furin-generated hNotch1 correlated with losses in NICD generation and Notch reporter activation, providing a direct link between heterodimer dissociation, NECD transendocytosis, and Notch activation. Based on our findings, we suggest that receptor dissociation by ligand cells serves to convert hNotch1 from an ADAM-insensitive substrate into one that is readily cleaved. In this regard, the heterodimeric nature of Notch is unique among ADAM substrates, whereas the membrane-bound subunit is similar in structure to conventional ADAM substrates (Schlondorff and Blobel, 1999). In fact, we show that the constitutive signaling activity intrinsic to a recombinant S1 form is dependent on ADAM cleavage, underscoring the role of the NECD in protecting the membrane-spanning subunit from proteolytic activation in the absence of ligand. Studies have demonstrated that ADAM cleavage is a prerequisite for γ-secretase processing (Brou et al., 2000; Mumm et al., 2000), and an inverse relationship between the size of the ectodomain and the efficiency of γ-secretase cleavage has been proposed (Struhl and Adachi, 2000). Therefore, we conclude that the ADAM requirement in Notch signaling reflects a role for this protease in trimming the extracellular sequences of the membrane-bound subunit, which would allow efficient γ-secretase cleavage to produce appropriate levels of NICD for biological responses.
Is DSL ligand–mediated endocytosis the force behind Notch heterodimer dissociation?
We found that endocytic-defective ligands were unable to efficiently dissociate hNotch1 and activate Notch signaling. Interestingly, losses in ligand endocytosis that perturb signaling did not diminish receptor binding or clustering, which is consistent with our previous report that ligand binding is not sufficient to induce Notch signaling (Yang et al., 2005). We demonstrate that in addition to ligand binding, productive Notch signaling requires receptor dissociation that is promoted by ligand endocytosis. Although a low level of signaling was detected when ligand endocytosis was defective, this activity may reflect cell detachment and/or Notch endocytosis, which could produce sufficient force for receptor dissociation. In fact, prefixed Delta-expressing S2 cells (that are presumably endocytosis defective) have also been reported to activate Notch target genes (Mishra-Gorur et al., 2002). Moreover, soluble DSL ligands are not as potent as cell-associated ligands (Shimizu et al., 2002) and must be preclustered (Wang et al., 1998; Morrison et al., 2000; Hicks et al., 2002) and/or immobilized (Varnum-Finney et al., 2000). Although it is unknown whether endocytosis, by itself, could provide such a physical force, both the actin cytoskeleton and dynamin have been implicated in inducing membrane constriction and tension during the process of endocytosis (Itoh et al., 2005; Roux et al., 2006).
In summary, this study has defined a direct role for NECD transendocytosis by DSL ligand cells in the activation of Notch signaling, and established a system to study the underlying mechanisms. In contrast to current models, we found that NECD transendocytosis occurs independent of ADAM proteolysis and requires separation of the hNotch1 subunits, which are driven not by ligand binding, but by ligand endocytosis. Previous studies have proposed that endocytosis of ligand–receptor complexes produces a change in Notch receptors that promotes proteolytic activation (Parks et al., 2000). Our findings suggest that physical separation of the Notch heterodimer is the structural change induced by ligand endocytosis, and we further speculate that ligand endocytosis acts via a mechanical force to first disrupt the noncovalently attached hNotch subunits, and that dissociation is required for proteolytic activation. Our model proposes that a critical event in Notch signaling is nonenzymatic dissociation of the Notch receptor, bringing Notch activation closer to the realm of mechanotransduction than previously proposed proteolytic cleavage models.
Materials and methods
Cell culture and plasmids
Stable Dll1, J1, and N1 cell lines were generated and cultured as previously described (Hicks et al., 2000; Bush et al., 2001; Ladi et al., 2005; Yang et al., 2005). The cDNA constructs used were as follows: pBos-rDll1, pBos-rDll1ΔICD (rDll1 truncated at D573), pBos-rDll10CD (rDll1 truncated at V560), pBos-HA-rDll1 (three tandem HA epitopes inserted between P536 and W537 of rDll1), pBos-rDll1-HA (three tandem HA epitopes inserted after V714), pBos-HA-rN1 (three tandem HA epitopes inserted between R23 and C24 of rN1), pBos-HA-rN1ΔFC (36 aa deletion from R1628 to H1663), pBos-HA-rN1-EGFP (EGFP inserted after K2561 of HA-rN1), pBos-HA-rN1ΔFC-EGFP (EGFP inserted after K2525 of HA-rN1ΔFC), pcDNA3.1-Dyn1K44A, pEGFP(N1)-Dyn1K44A, pEGFP(C2)-Eps15DIII (gifts from S. Schmid, The Scripps Research Institute, La Jolla, CA), and pEGFP(N2; CLONETCH Laboratories, Inc.). pCDNA3-p120 (a gift from J. Aster, Brigham and Women's Hospital, Boston, MA) mimics the furin cleavage fragment of human N1 (M1-R23 joined by a BamHI linker to E1666-K2555). Potential translational initiation codons (AUG, CUG, and GUG) found upstream of the S3/γ-secretase site were modified via a PCR strategy to produce pCDNA3-p120mis (mutated initiation sites). Wobble mutations were introduced into CUG and GUG codons to maintain the amino acid sequence; however, AUG methionine residues were changed to valine. The amino acid positions affected, as well as the respective altered codon sequences, are as follows: Leu1667(CTT), Met1670Val(GTA), Val1700(GTA), Val1722(GTT), Val1727(GTA), Leu1735(CTT), Met1738Val(GTA), Val1740(GTA), Val1746(GTT), Leu1748(CTA), Val1751(GTA), Val1755(GTT), Leu1756(CTT), and Leu1757(CTA). Sequencing confirmed that only intended substitutions were introduced. For p120misΔmyc, the C terminus of p120mis from M2094-K2555 was replaced with six tandem myc epitope tags. Cloning details are available upon request.
Immunoprecipitation and immunoblotting
C2C12 stable cell lines were cell surface biotinylated with Sulfo-NHS-Biotin. Biotinylated proteins were isolated with SAV-immobilized beads after quantification and equilibration. The SAV precipitates were analyzed by Western blotting with 93–4 serum or OPA1 antibodies (BD Biosciences). COS7 cells were transfected with p120mis or p120misΔmyc using Lipofectamine (Invitrogen). Immediately after transfection, the cells were treated either with 3 μM BB94, 10–20 μM DAPT, or DMSO for 22 h, with inclusion of 2–10 μM MG132 for the last 5 h. Cell lysates were either collected directly into 2xSB + 100 μM DTT for Western blotting with anti-myc (9E10), anti–α-tubulin, and anti-Val1744 antibodies or immunoprecipitated with antibodies to N1 intracellular domain (PCR12), followed by Western blotting with antibodies to N1 intracellular domain (93–4) or antibodies to activated NICD (Val 1744).
Staining and immunofluorescence labeling
The primary antibodies used were as follows: rabbit polyclonal anti–extracellular domain Dll1 (148G), 1:500; rabbit polyclonal anti–extracellular domain J1 (PCR8), 1:500; mouse anti-HA (262K), 1:1,000; rabbit anti-cleaved N1 (Val1744), 1:1,000 (Cell Signaling Technology); mouse anti-HA conjugated to Alexa Fluor 488 (16B12), 1:1,000; mouse anti-GFP (3E6), 1:1,000; rabbit anti-GFP conjugated to Alexa Fluor 488, 1:1,000; (Invitrogen). The secondary antibodies used were as follows: goat anti–rabbit conjugated to Alexa Fluor 633; goat anti–rabbit conjugated to Alexa Fluor 568; goat anti–rabbit conjugated to Alexa Fluor 488; goat anti–mouse conjugated to Alexa Fluor-568; and goat anti–mouse conjugated to Alexa Fluor 488, all at 1:1,000 (Invitrogen). Cells were fixed in PBS containing 4% formaldehyde and 4% sucrose for 10 min; aldehydes were quenched with 50 mM ammonium chloride in PBS for 15 min at room temperature, permeabilized with 0.1% Triton X-100 for 5 min, blocked in staining buffer (10% goat serum, 1% BSA, and 0.05% sodium azide in PBS) for 1 h, and incubated with antibodies diluted in staining buffer for 2 h or overnight. Samples were mounted in ProLong Gold (Invitrogen), and images were acquired at room temperature on an LSM 5 PASCAL laser-scanning microscope equipped with a Plan-Neofluar 100×/1.3 NA objective (both from Carl Zeiss MicroImaging, Inc.). Within each experiment, instrument settings (laser intensity, gain) were kept constant. Images were acquired and analyzed using LSM PASCAL software. Where appropriate, images are projections of several confocal sections.
Image quantification
For analysis of transendocytosis, suspensions of C2C12 cells stably expressing HA-N1 were obtained by trituration and incubated for 45 min at 37°C with DSL ligand L cells preseeded overnight on glass coverslips. Cocultures were initially examined under red channel fluorescence, and interactions displaying multiple DSL-induced N1 surface puncta ≥0.5 μm in size were assayed for presence of a green signal within the interacting DSL ligand cell. L cells do not induce N1 puncta, and thus were not quantitated. 120–150 cells were assayed, and the percentage of interacting cells with an intracellular green signal was determined.
N1 N-terminal dissociation was measured from triturated HA-N1-EGFP cells cocultured for 45 min at 37°C with DSL ligand L cells preseeded overnight on glass coverslips. Image acquisition parameters were set such that fluorescent signals were within the linear range of a 12-bit image, and projections of the maximum Z-series were collected. Transmitted light channel was used to designate regions of interest that outlined interacting DSL ligand and Notch-expressing cells. After thresholding, fluorescence area from each channel was quantitated for regions of interest. Dissociation ratios were determined by dividing the area of red channel fluorescence by the area of green channel fluorescence for more than three fields per experiment.
Percentage of NICD-positive nuclei was determined after a 4-h coculture of triturated HA-N1 cells with DSL ligand cells in the presence of 10 μM of the proteosome inhibitor MG132 (BIOMOL) to allow NICD accumulation. Cocultures were initially examined under green channel fluorescence, and N1 cells displaying multiple HA-positive clusters ≥0.5 μm in size were then assayed for nuclear NICD immunoreactivity, and the percentage of NICD-positive nuclei was calculated for 120–150 N1 cells.
C2C12 N1 cells were treated with 5 μM BB94 (British Biotechnology) to inhibit metalloproteases, 50 μM DAPT (Calbiochem) to inhibit γ-secretase, or DMSO as the vehicle control.
Dll1 cells were transfected with EGFP, dynK44A-EGFP, or EGFP-Eps15DIII using Transfast (Promega) and cocultured with HA-N1 C2C12 cells. Green channel fluorescence was used to select 100–120 EGFP-positive Dll1 cells, which were scored for a vesicular HA-N1 signal. The percentage of Dll1 cells exhibiting N1 transfer was determined.
For all image quantification, data collected from three independent experiments were used to calculate the mean, SEM, and P value from unpaired t tests.
Binding and uptake of soluble Dll1, N1, and antibody
Dll1Fc binding to HEK 293T cells transfected with HA-N1 or HA-N1ΔFC was analyzed by flow cytometry (FACSCalibur; Becton Dickinson), as previously described (Ladi et al., 2005; Yang et al., 2005). Dll1 cells were incubated with soluble N1Fc (R&D Systems) preclustered with goat anti–human Fc (1:150; Jackson ImmunoResearch Laboratories) conjugated to FITC or Texas red and coincubated with transferrin-polylysine-FITC conjugate (Sigma-Aldrich) at 37°C before fixation and analysis. For antibody uptake, cells were incubated with 148G (1:2,000) before fixation, permeabilization, and detection with goat anti–rabbit secondary antibodies. Cell-surface staining of HA was performed on HA-N1-EGFP or HA-N1ΔFC-EGFP C2C12 cells on ice with anti-HA antibody (16B12) and goat anti–mouse conjugated to APC (Invitrogen) and analyzed by flow cytometry.
Reporter assays
Ligand-induced signaling measured by the CSL-luciferase Notch-responsive reporter were assayed using either parental L cells or stable cell lines expressing Dll1, Dll1ΔICD, or HEK293T cells transfected with Dll1 plus EGFP, Dll1 plus dynK44A-EGFP, or Dll1 plus EGFP-Eps15DIII, in the presence or absence of 5 μM BB94, 50 μM DAPT, or DMSO assayed using the Dual-Luciferase Reporter Assay System (Promega), as previously described (Hicks et al., 2000; Ladi et al., 2005; Yang et al., 2005). Ligand-independent signaling was measured in COS7 cells transfected with Lipofectamine (Invitrogen), as described by Miyamoto et al. (2006). The lipofection mix was removed after 5 h and replaced by DME supplemented with 1% FBS and 3 μM BB94, 10 μM DAPT, or DMSO. Cells were collected after 4–5 h for luciferase assays that were performed using the Dual-Luciferase Reporter Assay System. The assay was performed twice in triplicate.
Online supplemental material
Fig. S1 shows coendocytosis of Dll1 and Notch1 N terminus by Dll1 cells. Fig. S2 displays ligand-induced Notch cell activation and NICD nuclear translocation. Fig. S3 exemplifies the methodology for calculation of Notch1 dissociation ratio. Fig. S4 illustrates that deletion of the furin cleavage site in Notch1 permits surface expression and ligand binding. Fig S5 shows that inhibition of Dll1-specific, or general endocytosis does not reduce Dll1 surface expression or Notch1 binding. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200609014/DC1.
Acknowledgments
We thank Kelsey Martin for help and advice on confocal microscopy and Jim Boulter, Greg Payne, Ellen Robey, and Larry Zipursky for helpful comments. We also thank Sandra Schmid for dynaminK44A-GFP and Eps15DIII-EGFP constructs, Jon Aster for the S1 construct, and British Biotechnology for BB94. We acknowledge the generation of Dll1 antiserum (148G) and Dll1ΔICD construct by Guy diSibio, J1 antiserum (PCR8) by Carol Hicks, N1ΔFC construct by L.T. Yang, and D1-10 cells by Maria Escriva. Flow cytometry was performed at the University of California Los Angeles Jonsson Comprehensive Cancer Center Flow Cytometry Core Facility, which is supported by National Institutes of Health grants NIH-CA-16042 and AI-28697.
This work was supported by NIH grants NS31885 and NS049084, STOP CANCER (G. Weinmaster), and the Ruth L. Kirschstein National Research Service Awards GM07185 and F31 EB006278 (J.T. Nichols).
Abbreviations used in this paper: ADAM, a disintegrin and metalloprotease; CSL, CBF1, SuH, LAG-1; DIC, differential interference contrast; DSL, Delta/Serrate/Lag-2; hNotch, heterodimeric Notch; NECD, Notch extracellular domain; NICD, Notch intracellular domain; RIP, regulated intramembrane proteolysis.
References
- Benmerah, A., C. Lamaze, B. Begue, S.L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaumueller, C.M., H. Qi, P. Zagouras, and S. Artavanis-Tsakonas. 1997. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 90:281–291. [DOI] [PubMed] [Google Scholar]
- Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail Jr., A. Doedens, P. Cumano, B.R.A. Roux, and A. Israel. 2000. A novel proteolytic cleavage involved in Notch signaling: the role of the Disintegrin-Metalloprotease TACE. Mol. Cell. 5:207–216. [DOI] [PubMed] [Google Scholar]
- Brown, M.S., J. Ye, R.B. Rawson, and J.L. Goldstein. 2000. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 100:391–398. [DOI] [PubMed] [Google Scholar]
- Bush, G., G. diSibio, A. Miyamoto, J.B. Denault, R. Leduc, and G. Weinmaster. 2001. Ligand-induced signaling in the absence of furin processing of Notch1. Dev. Biol. 229:494–502. [DOI] [PubMed] [Google Scholar]
- Cagan, R.L., H. Kramer, A.C. Hart, and S.L. Zipursky. 1992. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 69:393–399. [DOI] [PubMed] [Google Scholar]
- Chitnis, A. 2006. Why is delta endocytosis required for effective activation of notch? Dev. Dyn. 235:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke, H., T. Baba, D.E. Warnock, and S.L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, C., S.H. Johnston, G. diSibio, A. Collazo, T.F. Vogt, and G. Weinmaster. 2000. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat. Cell Biol. 2:515–520. [DOI] [PubMed] [Google Scholar]
- Hicks, C., E. Ladi, C. Lindsell, J.J. Hsieh, S.D. Hayward, A. Collazo, and G. Weinmaster. 2002. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J. Neurosci. Res. 69:60–71. [DOI] [PubMed] [Google Scholar]
- Itoh, T., K.S. Erdmann, A. Roux, B. Habermann, H. Werner, and P. De Camilli. 2005. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell. 9:791–804. [DOI] [PubMed] [Google Scholar]
- Klueg, K.M., and M.A. Muskavitch. 1999. Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: members of the Notch signalling pathway in Drosophila. J. Cell Sci. 112:3289–3297. [DOI] [PubMed] [Google Scholar]
- Klueg, K.M., T.R. Parody, and M.A. Muskavitch. 1998. Complex proteolytic processing acts on Delta, a transmembrane ligand for Notch, during Drosophila development. Mol. Biol. Cell. 9:1709–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladi, E., J.T. Nichols, W. Ge, A. Miyamoto, C. Yao, L.T. Yang, J. Boulter, Y.E. Sun, C. Kintner, and G. Weinmaster. 2005. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J. Cell Biol. 170:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne, R., and F. Schweisguth. 2003. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell. 5:139–148. [DOI] [PubMed] [Google Scholar]
- Le Borgne, R., A. Bardin, and F. Schweisguth. 2005. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 132:1751–1762. [DOI] [PubMed] [Google Scholar]
- Lieber, T., S. Kidd, E. Alcamo, V. Corbin, and M.W. Young. 1993. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 7:1949–1965. [DOI] [PubMed] [Google Scholar]
- Lindsell, C.E., C.J. Shawber, J. Boulter, and G. Weinmaster. 1995. Jagged: a mammalian ligand that activates Notch1. Cell. 80:909–917. [DOI] [PubMed] [Google Scholar]
- Logeat, F., C. Bessia, C. Brou, O. LeBail, S. Jarriault, N. Seiday, and A. Israel. 1998. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA. 95:8108–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston, D.J., S. Dickinson, and C.D. Nobes. 2003. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat. Cell Biol. 5:851–853. [DOI] [PubMed] [Google Scholar]
- Mishra-Gorur, K., M.D. Rand, B. Perez-Villamil, and S. Artavanis-Tsakonas. 2002. Down-regulation of Delta by proteolytic processing. J. Cell Biol. 159:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, A., R. Lau, P.W. Hein, J.M. Shipley, and G. Weinmaster. 2006. Microfibrillar proteins MAGP-1 and MAGP-2 induce Notch1 extracellular domain dissociation and receptor activation. J. Biol. Chem. 281:10089–10097. [DOI] [PubMed] [Google Scholar]
- Morel, V., R. Le Borgne, and F. Schweisguth. 2003. Snail is required for Delta endocytosis and Notch-dependent activation of single-minded expression. Dev. Genes Evol. 213:65–72. [DOI] [PubMed] [Google Scholar]
- Morrison, S.J., S.E. Perez, Z. Qiao, J.M. Verdi, C. Hicks, G. Weinmaster, and D.J. Anderson. 2000. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 101:499–510. [DOI] [PubMed] [Google Scholar]
- Mumm, J.S., E.H. Schroeter, M.T. Saxena, A. Griesemer, X. Tian, D.J. Pan, W.J. Ray, and R. Kopan. 2000. A ligand-induced extracellular cleavage regulates g-secretase-like proteolytic activation of Notch1. Mol. Cell. 5:197–206. [DOI] [PubMed] [Google Scholar]
- Parks, A.L., K.M. Klueg, J.R. Stout, and M.A. Muskavitch. 2000. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 127:1373–1385. [DOI] [PubMed] [Google Scholar]
- Rand, M.D., L.M. Grimm, S. Artavanis-Tsakonas, V. Patriub, S.C. Blacklow, J. Sklar, and J.C. Aster. 2000. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol. Cell. Biol. 20:1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebay, I., R.G. Fehon, and S. Artavanis-Tsakonas. 1993. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 74:319–329. [DOI] [PubMed] [Google Scholar]
- Roux, A., K. Uyhazi, A. Frost, and P. De Camilli. 2006. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 441:528–531. [DOI] [PubMed] [Google Scholar]
- Sanchez-Irizarry, C., A.C. Carpenter, A.P. Weng, W.S. Pear, J.C. Aster, and S.C. Blacklow. 2004. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol. Cell. Biol. 24:9265–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff, J., and C.P. Blobel. 1999. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 112:3603–3617. [DOI] [PubMed] [Google Scholar]
- Schroeter, E., J. Kisslinger, and R. Kopan. 1998. Notch1 signalling requires ligand-induced proteolytic release of the intracellular domain. Nature. 393:382–386. [DOI] [PubMed] [Google Scholar]
- Seugnet, L., P. Simpson, and M. Haenlin. 1997. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev. Biol. 192:585–598. [DOI] [PubMed] [Google Scholar]
- Shimizu, K., S. Chiba, T. Saito, T. Takahashi, K. Kumano, Y. Hamada, and H. Hirai. 2002. Integrity of intracellular domain of Notch ligand is indispensable for cleavage required for release of the Notch2 intracellular domain. EMBO J. 21:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl, G., and A. Adachi. 2000. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol. Cell. 6:625–636. [DOI] [PubMed] [Google Scholar]
- Struhl, G., K. Fitzgerald, and I. Greenwald. 1993. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 74:331–345. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney, B., L. Wu, M. Yu, C. Brashem-Stein, S. Staats, D. Flowers, J.D. Griffin, and I.D. Bernstein. 2000. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 113:4313–4318. [DOI] [PubMed] [Google Scholar]
- Wang, S., A.D. Sdrulla, G. diSibio, G. Bush, D. Nofziger, C. Hicks, G. Weinmaster, and B. Barres. 1998. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 21:63–75. [DOI] [PubMed] [Google Scholar]
- Weng, A.P., A.A. Ferrando, W. Lee, J.P. Morris, L.B. Silverman, C. Sanchez-Irizarry, S.C. Blacklow, A.T. Look, and J.C. Aster. 2004. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 306:269–271. [DOI] [PubMed] [Google Scholar]
- Wilkin, M.B., and M. Baron. 2005. Endocytic regulation of Notch activation and down-regulation (review). Mol. Membr. Biol. 22:279–289. [DOI] [PubMed] [Google Scholar]
- Wu, G., S. Lyapina, I. Das, J. Li, M. Gurney, A. Pauley, I. Chui, R.J. Deshaies, and J. Kitajewski. 2001. SEL-10 is an inhibitor of notch signaling that targets notch for ubiquitin-mediated protein degradation. Mol. Cell. Biol. 21:7403–7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L.T., J.T. Nichols, C. Yao, J.O. Manilay, E.A. Robey, and G. Weinmaster. 2005. Fringe glycosyltransferases differentially modulate Notch1 proteolysis induced by Delta1 and Jagged1. Mol. Biol. Cell. 16:927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer, M., A. Palmer, J. Kohler, and R. Klein. 2003. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 10:869–878. [DOI] [PubMed] [Google Scholar]