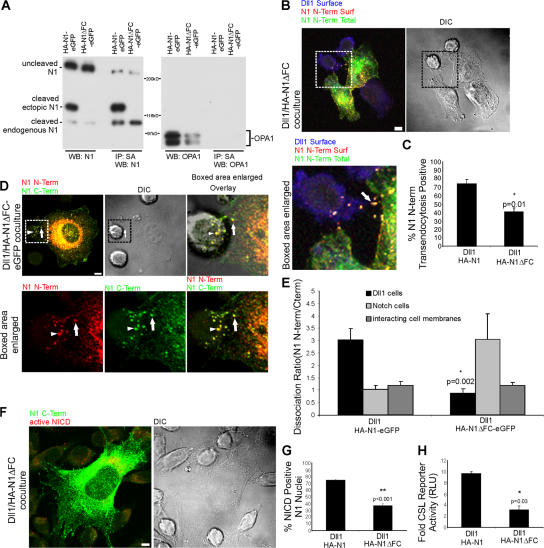
Figure 6.
Notch1 transendocytosis, N-terminal dissociation, and signaling require furin processing. (A) C2C12 cells stably expressing HA-N1-EGFP or the furin cleavage mutant HA-N1ΔFC-EGFP were biotinylated, and lysates were quantitated and equilibrated. Whole cell lysates, as well as streptavidin precipitates, were analyzed by Western blotting with antibodies to N1 intracellular domain (93–4), or OPA1. Uncleaved N1, as well as the C-terminal, 120-kD, furin-processed subunit of both ectopic and endogenous N1, are detected. (B) Dll1 cells were cocultured with HA-N1ΔFC cells, as in Fig. 2 C, to identify surface Dll1 (blue), surface N1 N terminus (red), and total N1 N terminus (green). Arrow indicates N1 N terminus at the interface of Notch and Dll1 cells. (C) Transendocytosis was quantified as in Fig. 2 D. (D) Dll1 cells were cocultured with HA-N1ΔFC-EGFP as in Fig. 4 A to identify N1 N terminus (red) and N1 C terminus (green). Arrows indicate double-positive N1 clusters at the interface of Notch and Dll1 cells; arrowheads denote double-positive structures associated with Dll1 cells. (E) Dissociation ratio was quantified for Dll1 cells, Notch cells, and the interacting cell membranes as in Fig. 4 B. (F) Dll1 cells were cocultured with HA-N1ΔFC cells and treated as in Fig. 3 A to identify N1 N terminus (green) and activated NICD (red). (G) NICD-positive nuclei were scored as in Fig. 3 B. (H) CSL reporter assay with HA-N1 or HA-N1ΔFC cells, as in Fig. 3 C. Cocultures in B, D, and F were imaged by confocal and DIC microscopy. Boxes indicate enlarged regions. Overlays are composites of fluorescent and DIC images. Error bars represent the SEM (C, E, and G) and the SD (H). *, P < 0.05, **, P < 0.001; t test relative to wild-type N1. Images from each experiment were uniformly adjusted using the levels function in Photoshop. Bars, 5 μm.