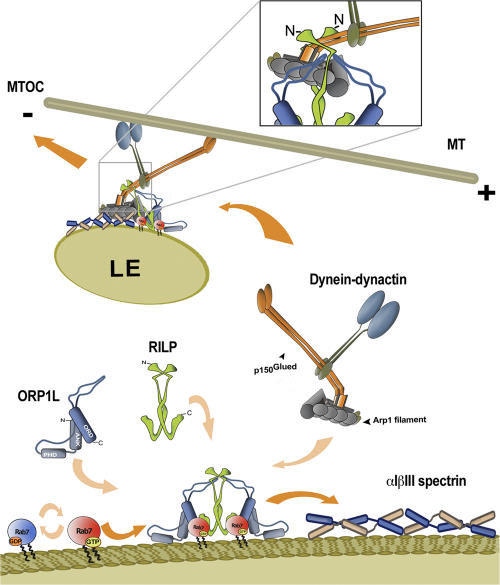
Figure 8.
Model for function of ORP1L in the translocation of the Rab7–RILP–p150Glued–dynein motor complex to βIII spectrin for minus-end transport. Active GTP-loaded Rab7 localizes to membranes of LEs and recruits the effectors RILP and ORP1L to form a dimer of a heterotrimeric complex. The C-terminal domain of RILP interacts with the switch and interswitch (RabSF1 and RabSF4) regions of Rab7, and the N-terminal half of RILP binds to the C-terminal domain of p150Glued (shown in detail in the box). Subsequently, the 2.4-MD dynein–dynactin motor is recruited by the 0.35-MD ORP1L–Rab7–RILP complex on LEs. ORP1L is required to direct the entire complex to the 0.6-MD αIβIII spectrin. The interaction is mediated by Arp1 binding to βIII spectrin. αIβIII spectrin localizes to several different compartments, but it is not sufficient on its own to recruit dynein motors. Microtubule-based, dynein motor–driven minus-end transport of LEs can only occur after the specific late endocytic dynein receptor Rab7–RILP has interacted with the general dynein membrane receptor βIII spectrin, a process that is facilitated by ORP1L.