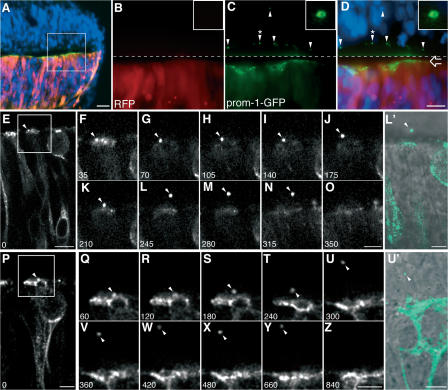
Figure 1.
Release of prom1-GFP–bearing particles from the apical surface of NE cells. Chick spinal cord (HH10–11) was coelectroporated with prom1-GFP and mRFP and analyzed 24 h later. (A–D) Transverse cryosection of fixed spinal cord analyzed by conventional fluorescence microscopy. DAPI is in blue, mRFP is in red, and prom1-GFP is in green. (A) Low-magnification overview. (B–D) Higher magnification of mRFP (B) and prom1-GFP (C) fluorescence in the area indicated by the white square in A; the merge in D includes the DAPI staining of nuclei. The top half of the images (i.e., above the dashed line) has been contrast enhanced for mRFP and prom1-GFP to facilitate detection of the prom1-GFP–bearing particles at the apical surface of, and within, the nontransfected, contralateral side of the neuroepithelium (arrowheads). The open arrow indicates the apical surface of transfected side of the neuroepithelium. The insets show a higher magnification of the prom1-GFP–bearing particle indicated by the arrowheads with asterisks. (E–Z) Slice cultures were subjected to time-lapse confocal imaging of prom1-GFP, using either 35-s (E–O) or 60-s (P–Z) intervals. The time points shown are indicated in the bottom left corner of each panel (in seconds). Selected single optical sections, following the prom1-GFP–bearing particle (arrowheads) through the z stack, are shown. The apical surface of the transfected side of the neuroepithelium is up. In E, note the basal position of the nucleus of the NE cell releasing the prom1-GFP–bearing particle. (F–O and Q–Z) Higher magnification of the area indicated by the white rectangle in E and P, respectively. (L′ and U′) Overlay of the DIC image and the prom1-GFP fluorescence (green) corresponding to L and U, respectively; note the localization of the prom1-GFP–bearing particle in the lumen of the neural tube (L′) and in the contralateral neuroepithelium (U′). Bars: (A) 20 μm; (D and E) 10 μm; (P, O, Z, L′, and U′) 5 μm.