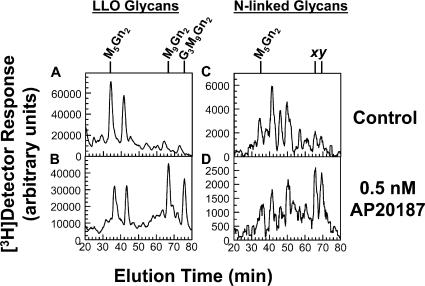
Figure 2.
PERK's kinase activity is sufficient to rectify LLO biosynthetic defects and aberrant N-linked glycosylation. Fv2E-PERK cells were cultured in the absence (A and C) or presence (B and D) of 0.5 nM AP20187 for 60 min. Incubation with or without AP20187 was then continued for an additional 20 min in medium containing 0.3 mM glucose (causing restricted LLO synthesis), 10% dialyzed FBS, and 40 μCi/ml [3H]mannose. [3H] LLO glycans (A and B) and [3H]N-linked glycans (C and D) were detected by HPLC. The positions of standards are indicated. x and y indicate N-linked glycans assigned the structures M9Gn2 and G1M9Gn2, respectively, and thus derived from G3M9Gn2-P-P-Dol rather than undermannosylated LLO intermediates (Shang and Lehrman, 2004c). Because of low [3H] labeling in N-glycan experiments, interference from spurious electronic noise was minimized by subjecting HPLC data to root-mean-square smoothing with PSI-Plot V.8 (Poly Software International).